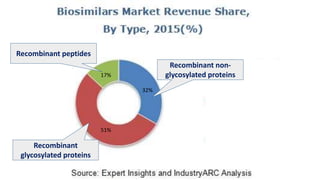
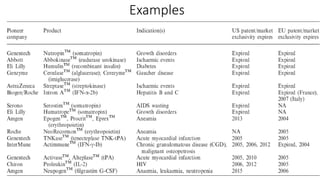
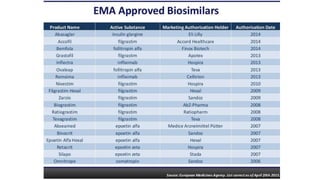
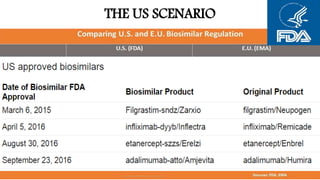
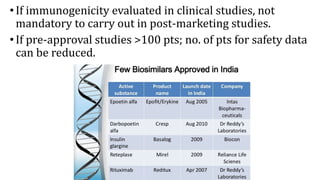
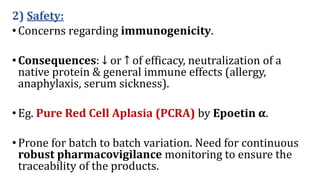
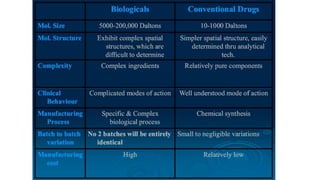
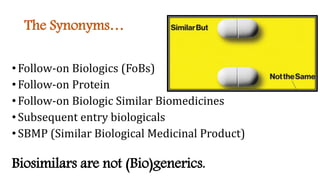
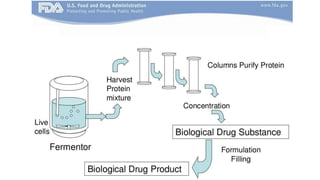
Biosimilars are biotherapeutic products that are similar to already approved reference biologics in terms of quality, safety and efficacy. They are developed to be highly similar but not identical to existing biologics. Regulatory agencies like EMA and FDA require extensive analytical, non-clinical and clinical studies including pharmacokinetic, immunogenicity and clinical efficacy trials to establish biosimilarity. While biosimilars could increase access and lower costs, issues related to efficacy, safety, substitution and labeling need to be addressed to ensure patient safety and appropriate use.





![[Calo-Fernández B, Martínez-Hurtado J (December 2012). "Biosimilars: Company Strategies to Capture Value
from the Biologics Market". Pharmaceuticals. 5 (12): 1393–1408]](https://image.slidesharecdn.com/biosimilars-dr-180309140030/85/Biosimilars-11-320.jpg)