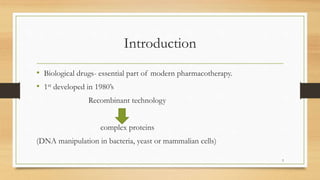
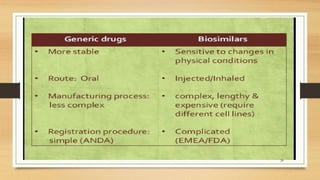
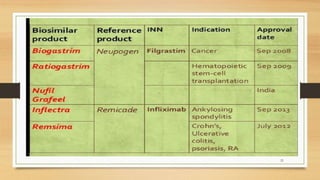
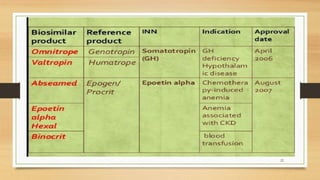
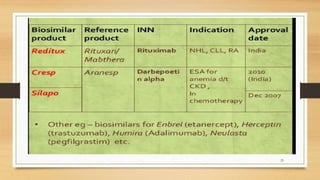
The document discusses biosimilars, which are biologic medicines that are similar but not identical to an original biologic. It describes the complex multi-step process used to develop and test biosimilars. This includes characterizing the original biologic, developing a unique cell line and process, testing for similarity through analytical and non-clinical studies, and clinical trials. Regulatory agencies oversee biosimilars differently than generics due to concerns over safety, substitution, naming, and labeling of the non-identical products.