Embed presentation
Download to read offline

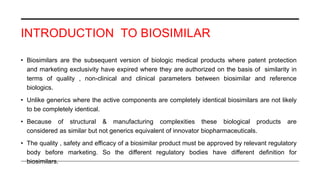

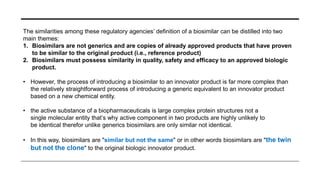

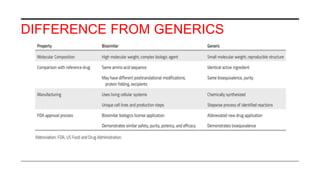
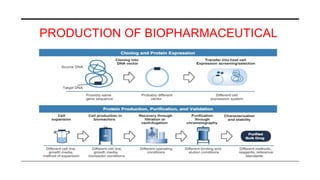
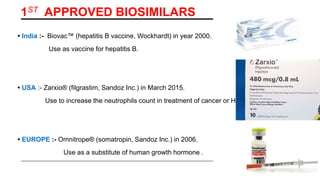







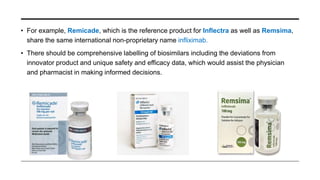


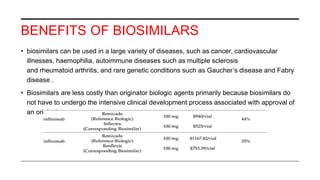



This document provides an overview of biosimilars. It defines biosimilars as subsequent versions of biologic medicines where patent protection has expired. Biosimilars are approved based on similarity to an original reference biologic in terms of quality, safety and efficacy, but are not expected to be identical due to structural complexities. The development of biosimilars involves extensive comparative studies to the reference product. Concerns with biosimilars include potential immunogenicity, efficacy issues, and uncertainty around switching between originator and biosimilar products or between biosimilars. Proper pharmacovigilance is important to monitor biosimilar safety and benefits.



























