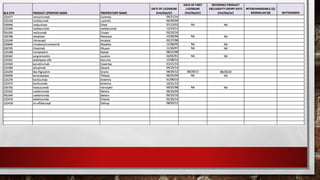
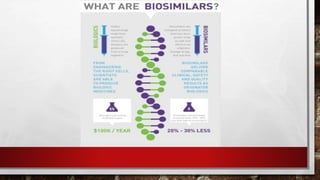
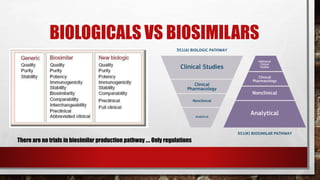
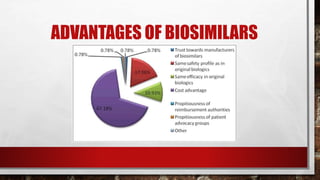
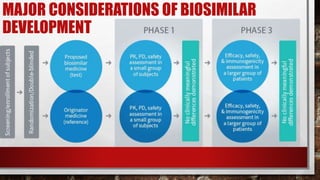
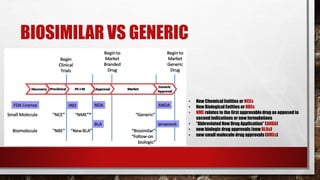
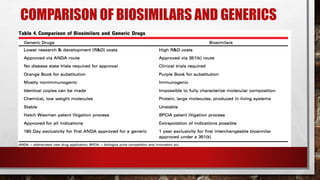
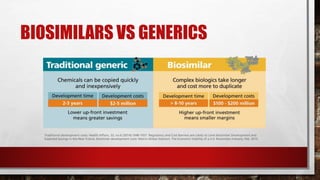
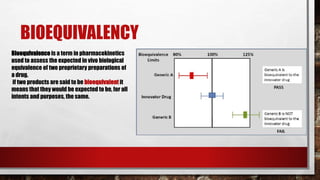
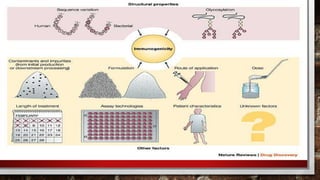
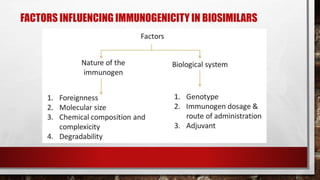
This document discusses biologics and biosimilars. It defines biologics as biological products made from natural sources like humans, animals or microorganisms that are used to treat or prevent diseases. Biosimilars are highly similar versions of biologics that are approved because they have no clinically meaningful differences. The document outlines key differences between biologics and biosimilars like regulatory pathways and development testing. It also compares biosimilars to generics and discusses important considerations for biosimilar development like immunogenicity, bioequivalence and post-translational modification.