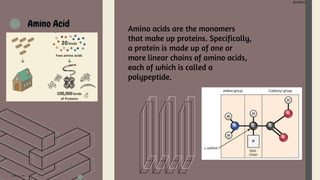
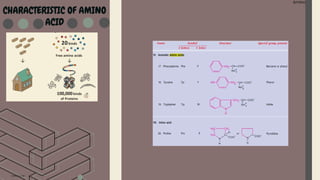
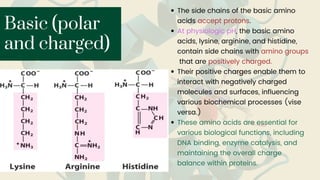
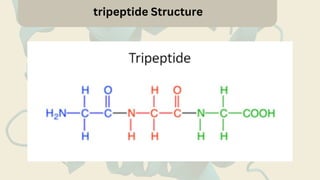
Amino acids are the building blocks of proteins. They contain an amino group, a carboxyl group, and a unique side chain. There are 20 standard amino acids used in protein construction. Amino acids can be classified based on the properties of their side chains as nonpolar, polar, or charged. Nonpolar amino acids have hydrophobic side chains and tend to be found in the interior of proteins. Polar and charged amino acids have hydrophilic side chains and are often found on the surface of proteins, where they can form hydrogen bonds and ionic interactions important for protein structure and function.