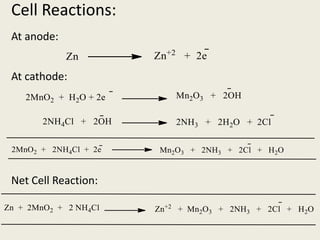
The document provides a detailed introduction to electrochemical energy systems, focusing on the classification of batteries into primary, secondary, and fuel cells. It explains the functioning, advantages, and disadvantages of various battery types, including dry cells, alkaline cells, ni-cd batteries, and fuel cells. Additionally, the document describes the chemical reactions involved in these batteries and their applications in everyday electronic devices.