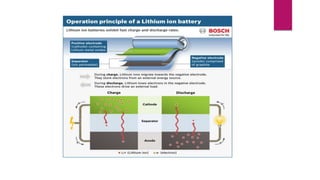
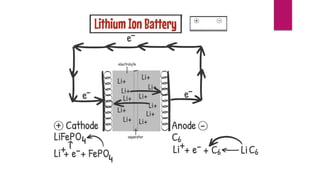
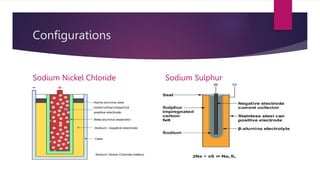
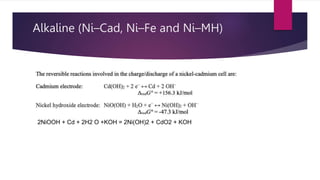
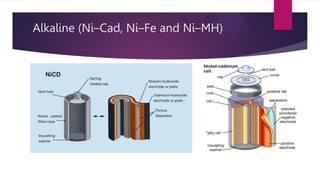
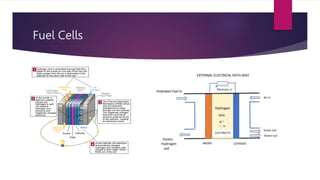
EV range is affected by cold weather and use of air conditioning and lights, which use battery energy. Most EV batteries are expected to last over 10 years, with capacity decreasing to 80% of original after that time. Lithium-ion batteries are commonly used in EVs due to their higher energy density and lower environmental impact compared to other battery technologies.