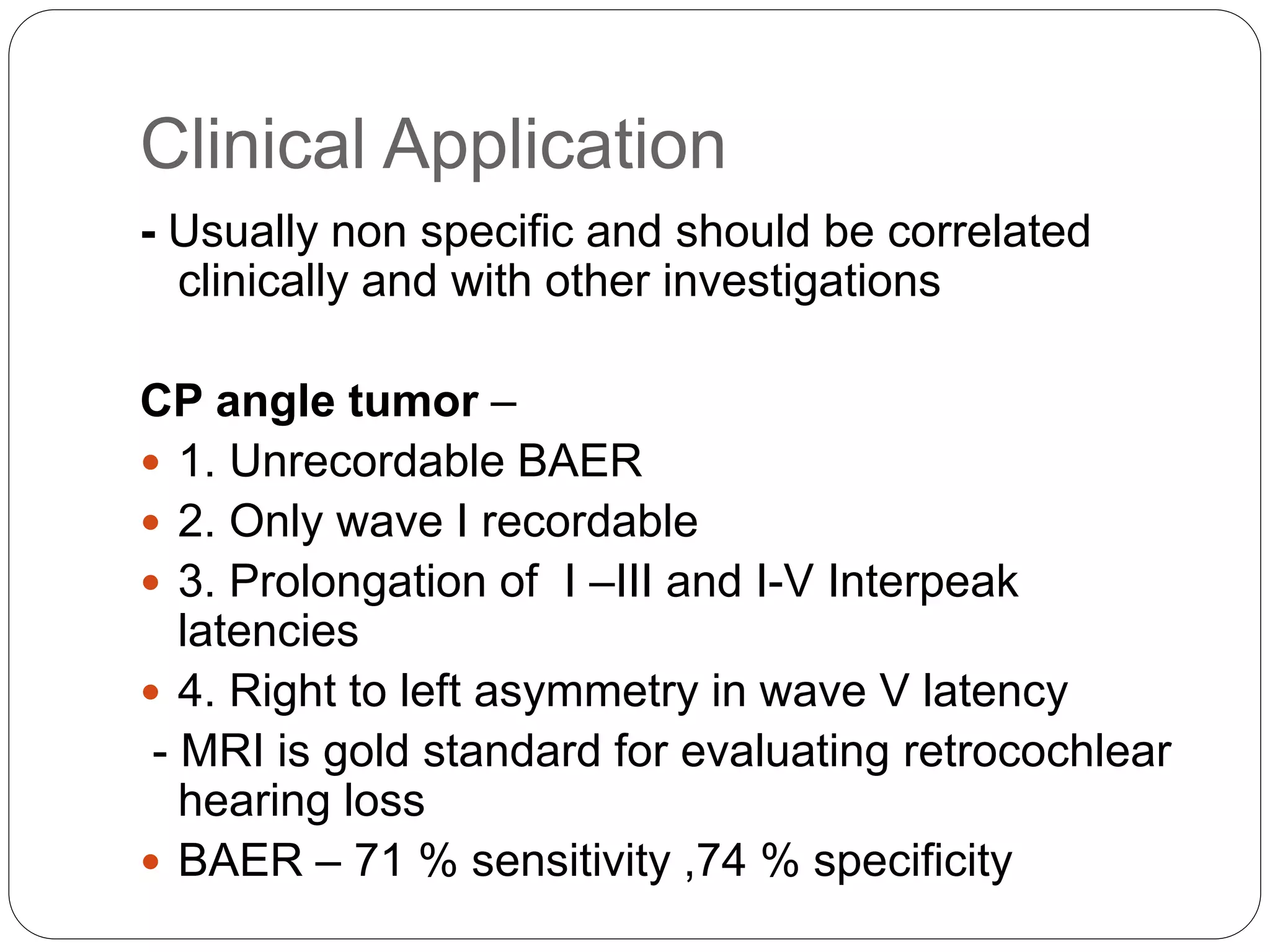
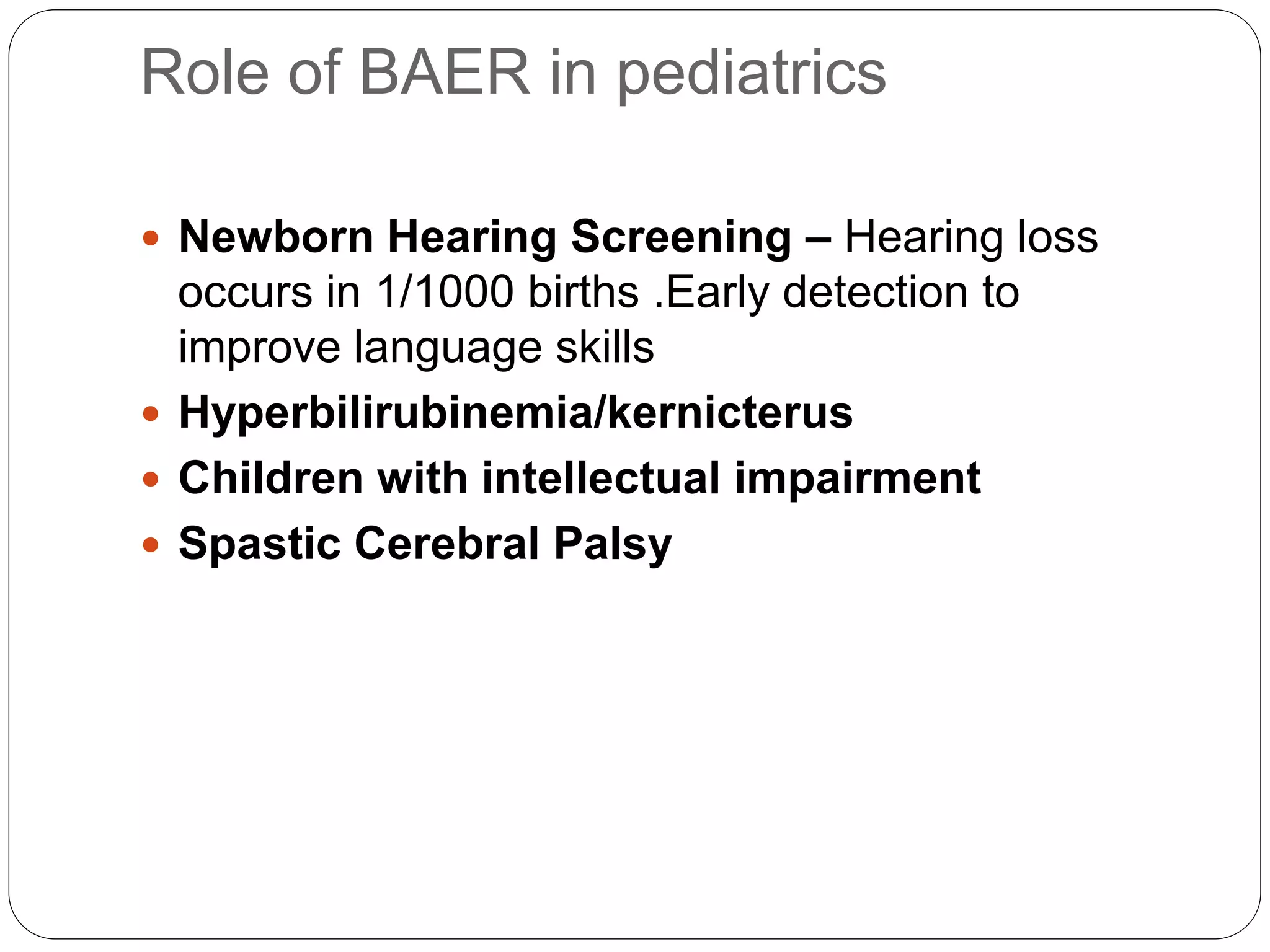
1. The document discusses various electrophysiological tests including repetitive nerve stimulation test (RNST), visual evoked potentials (VEP), and brainstem auditory evoked responses (BAER).
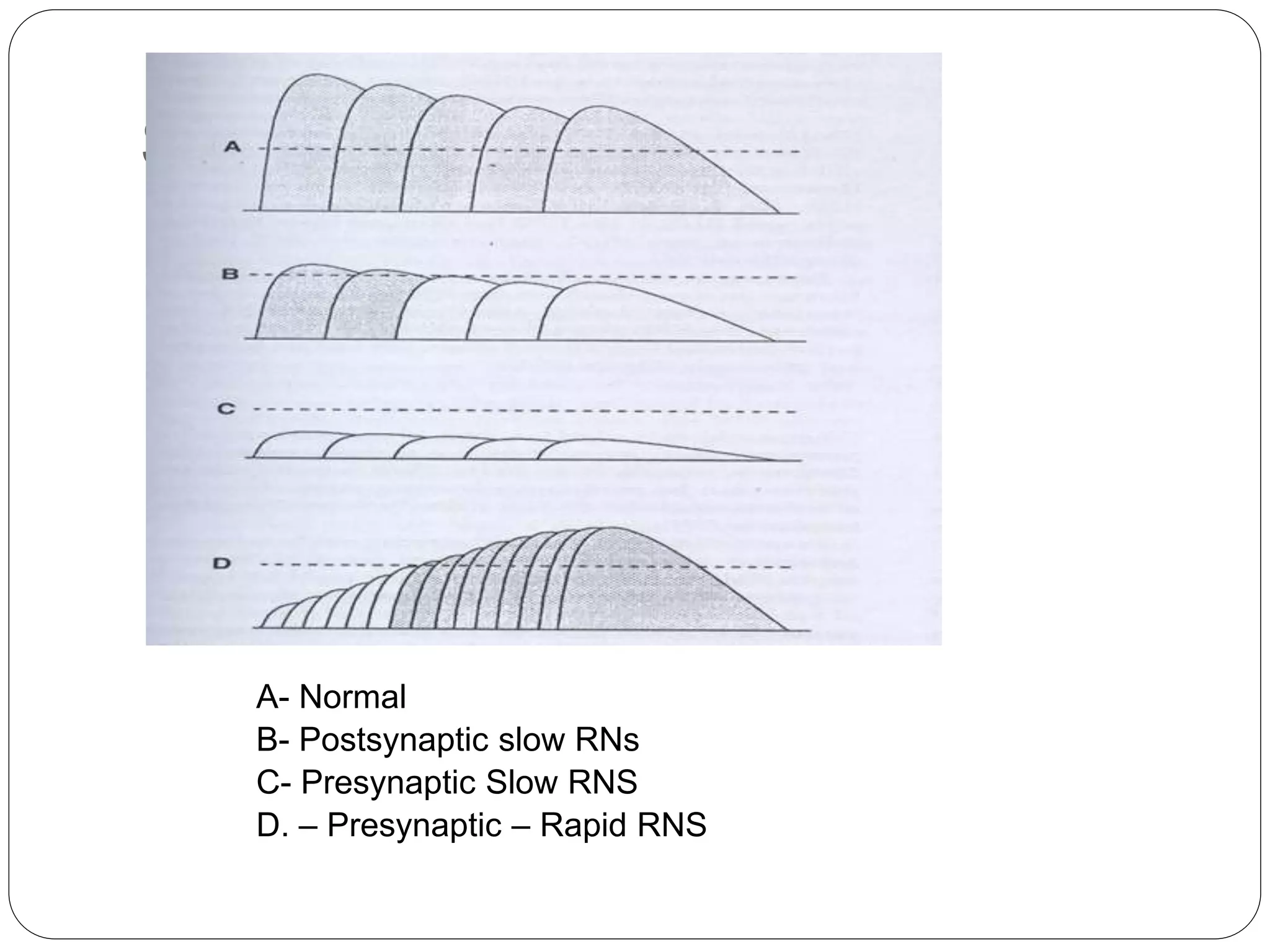
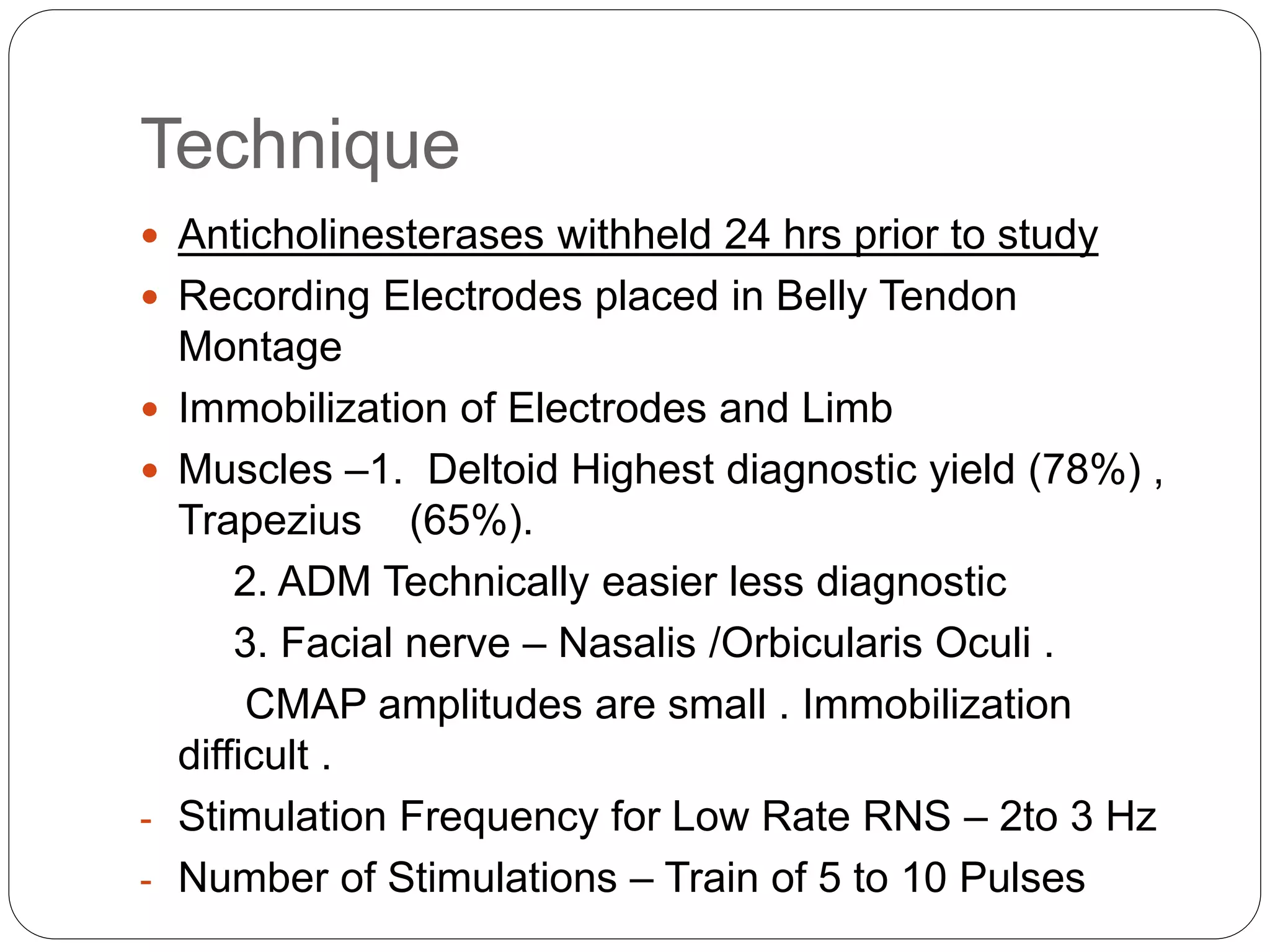
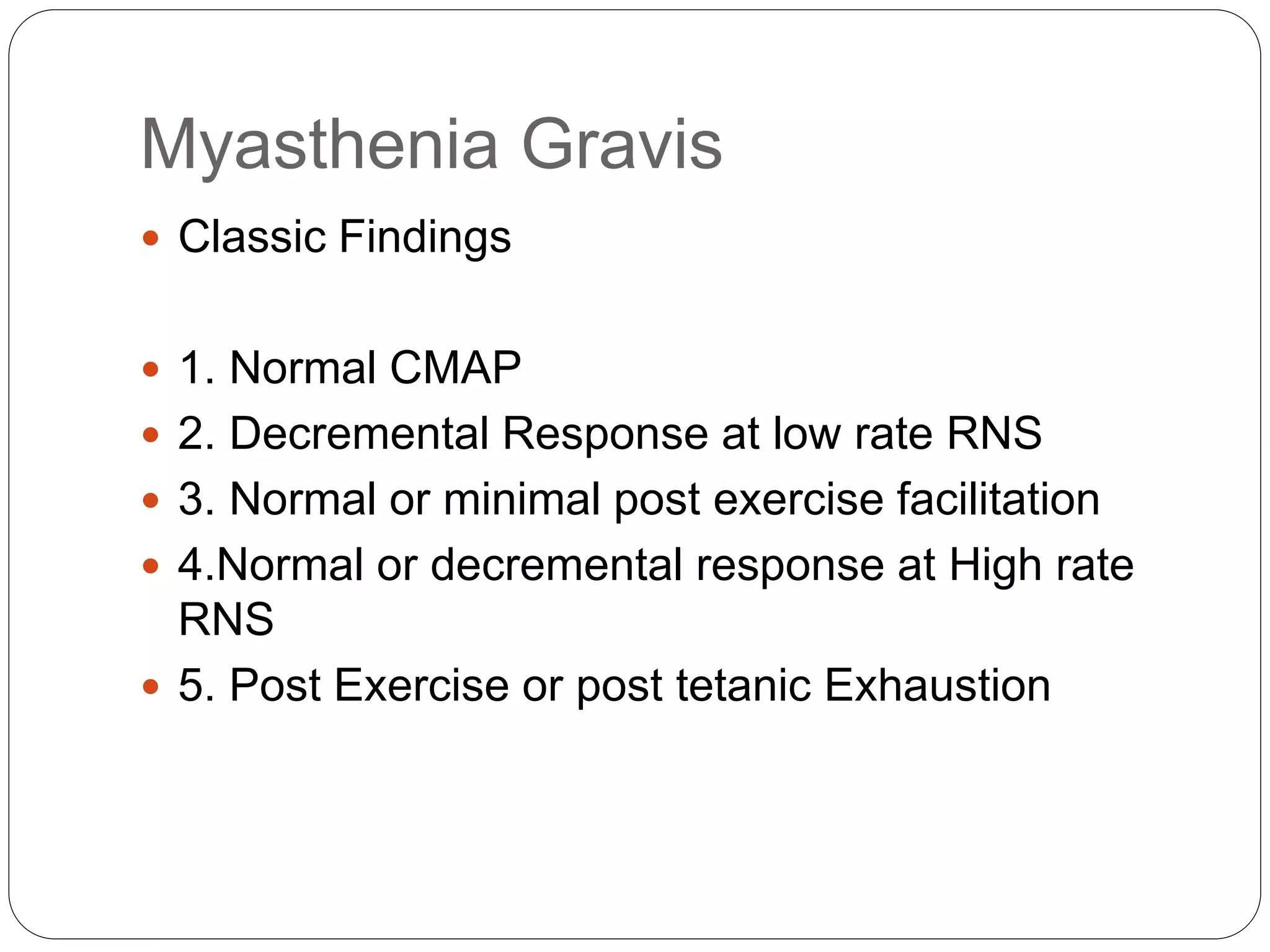
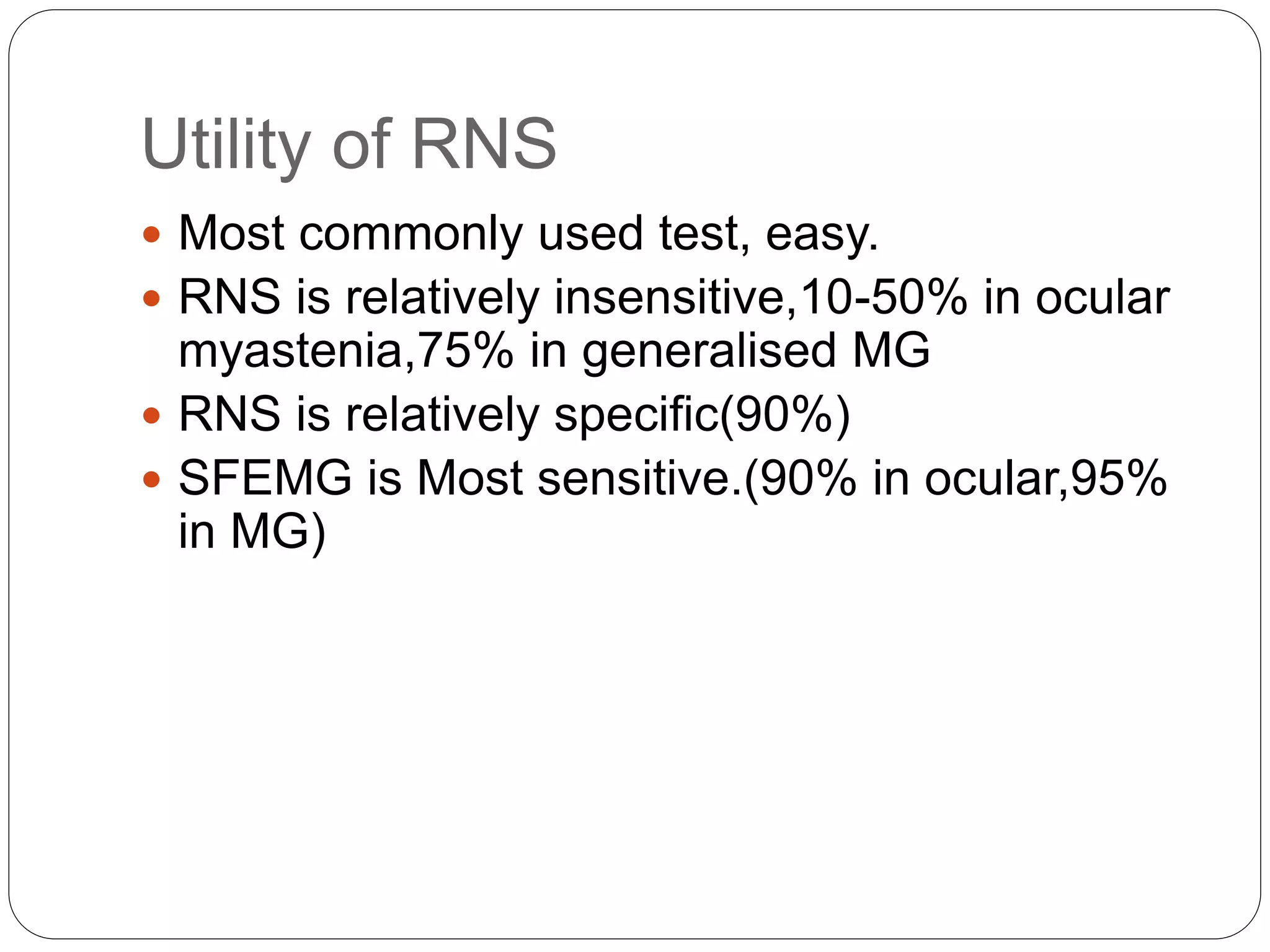
2. RNST involves electrically stimulating a motor nerve repeatedly to observe changes in the compound muscle action potential. Abnormal results can indicate disorders of the neuromuscular junction like myasthenia gravis or Lambert-Eaton myasthenic syndrome.
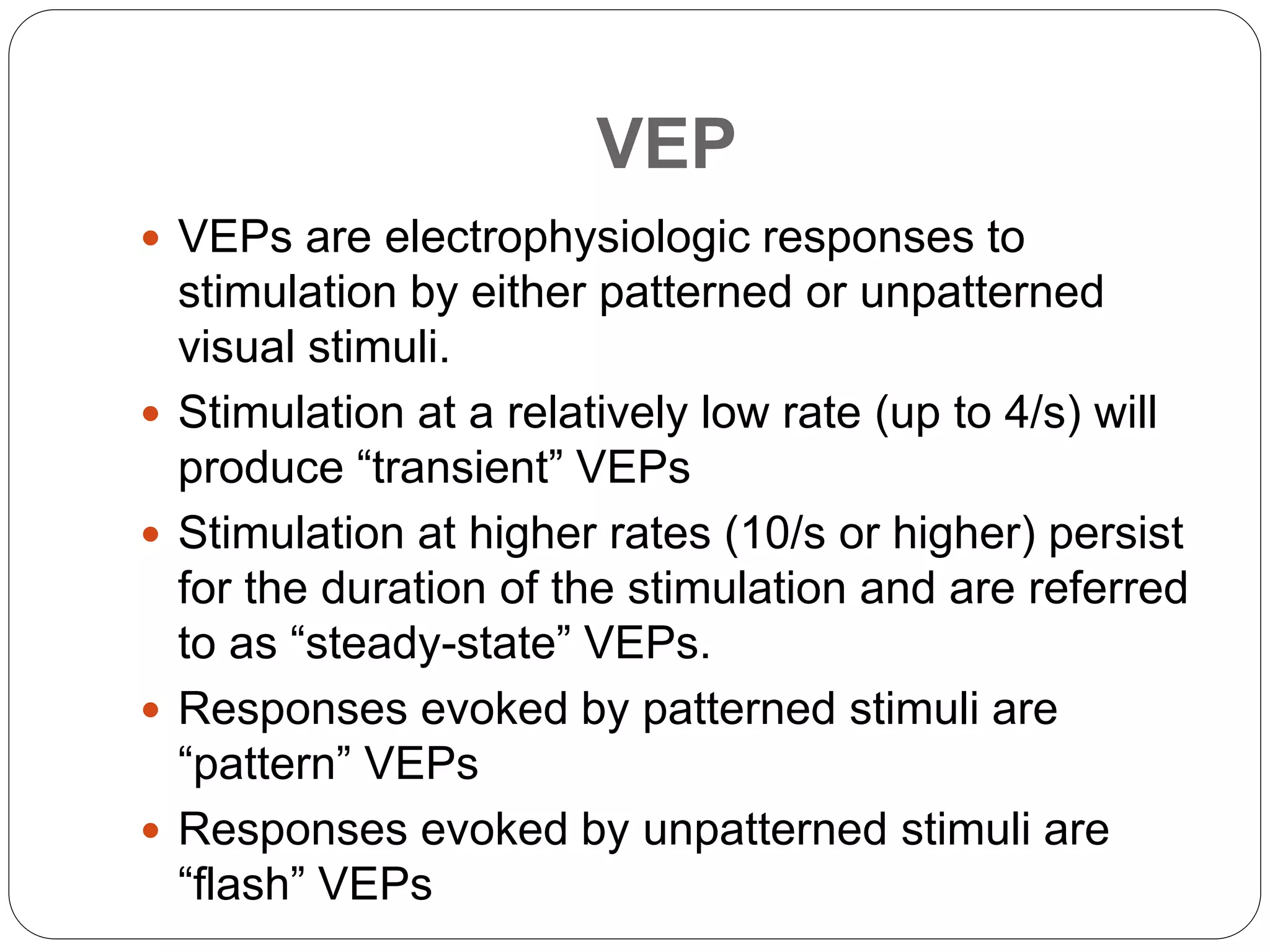
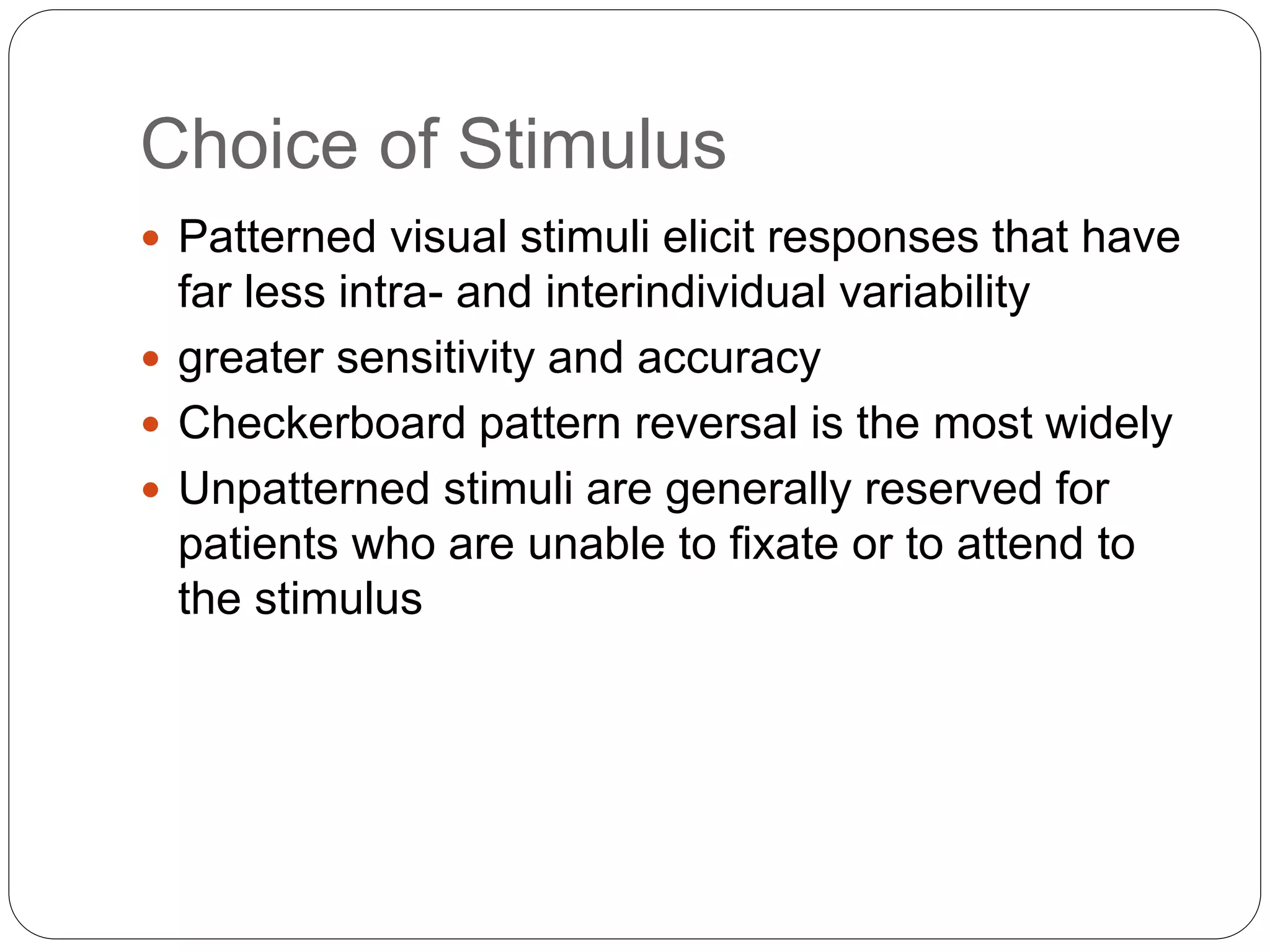
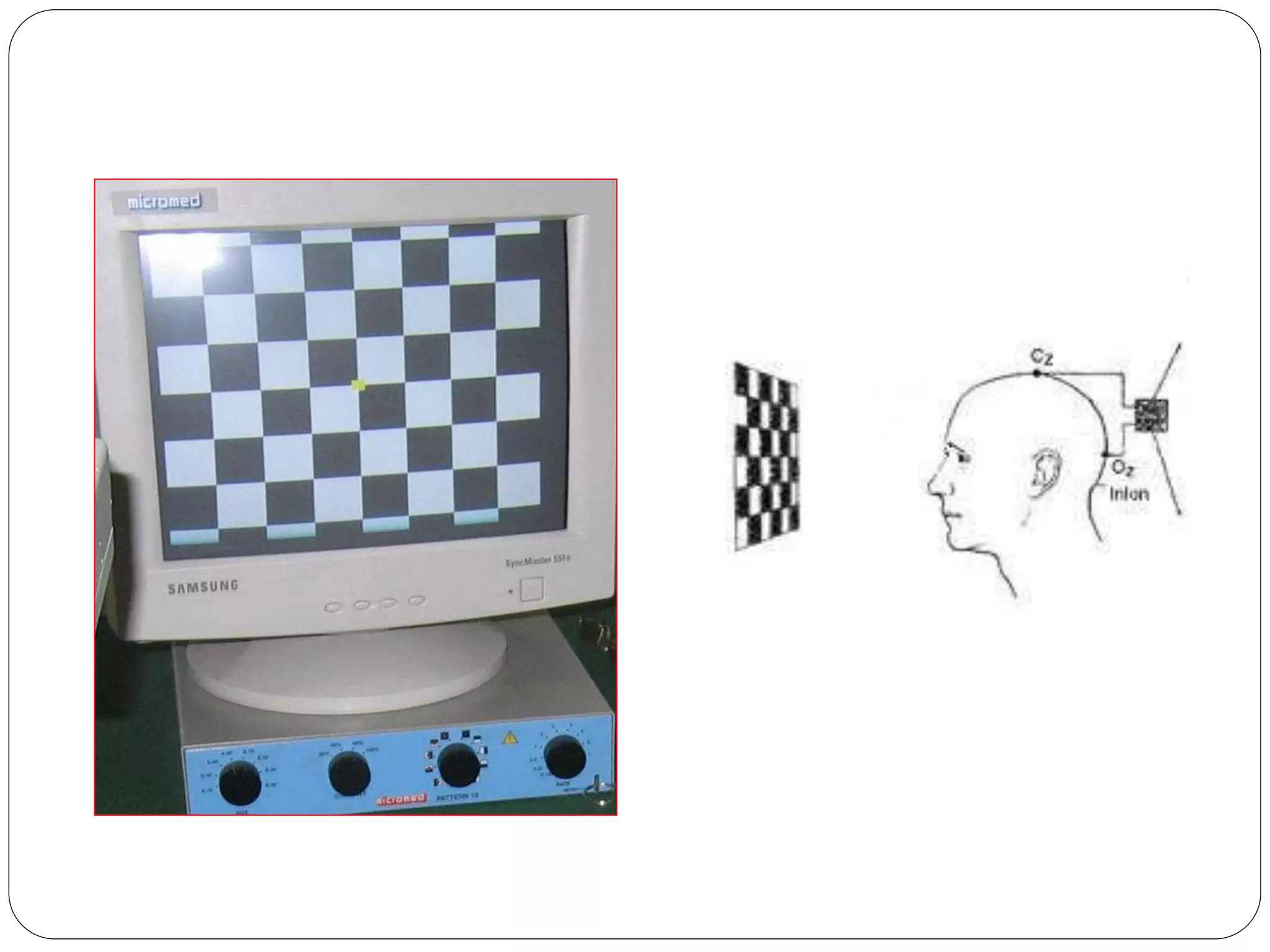
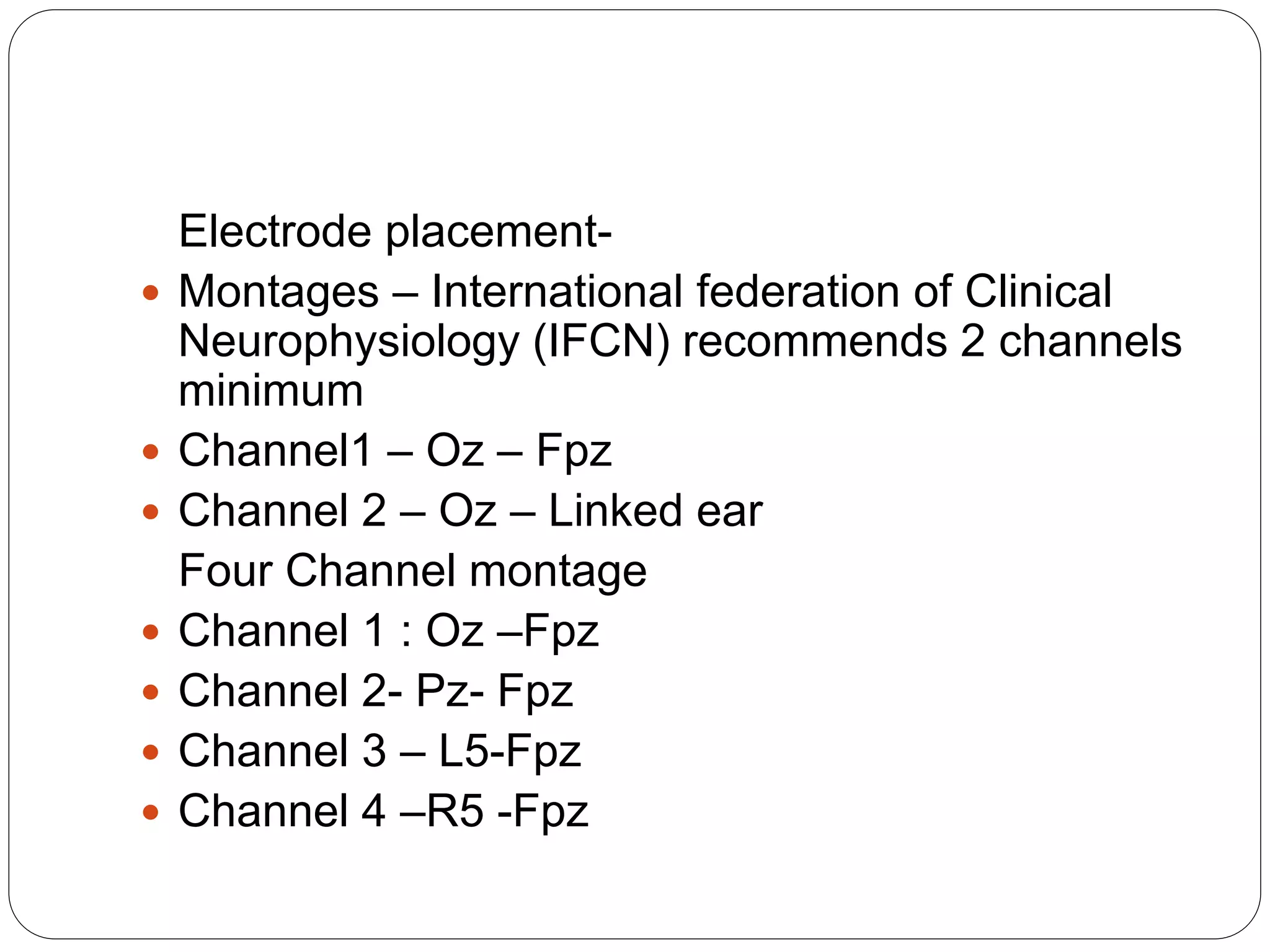
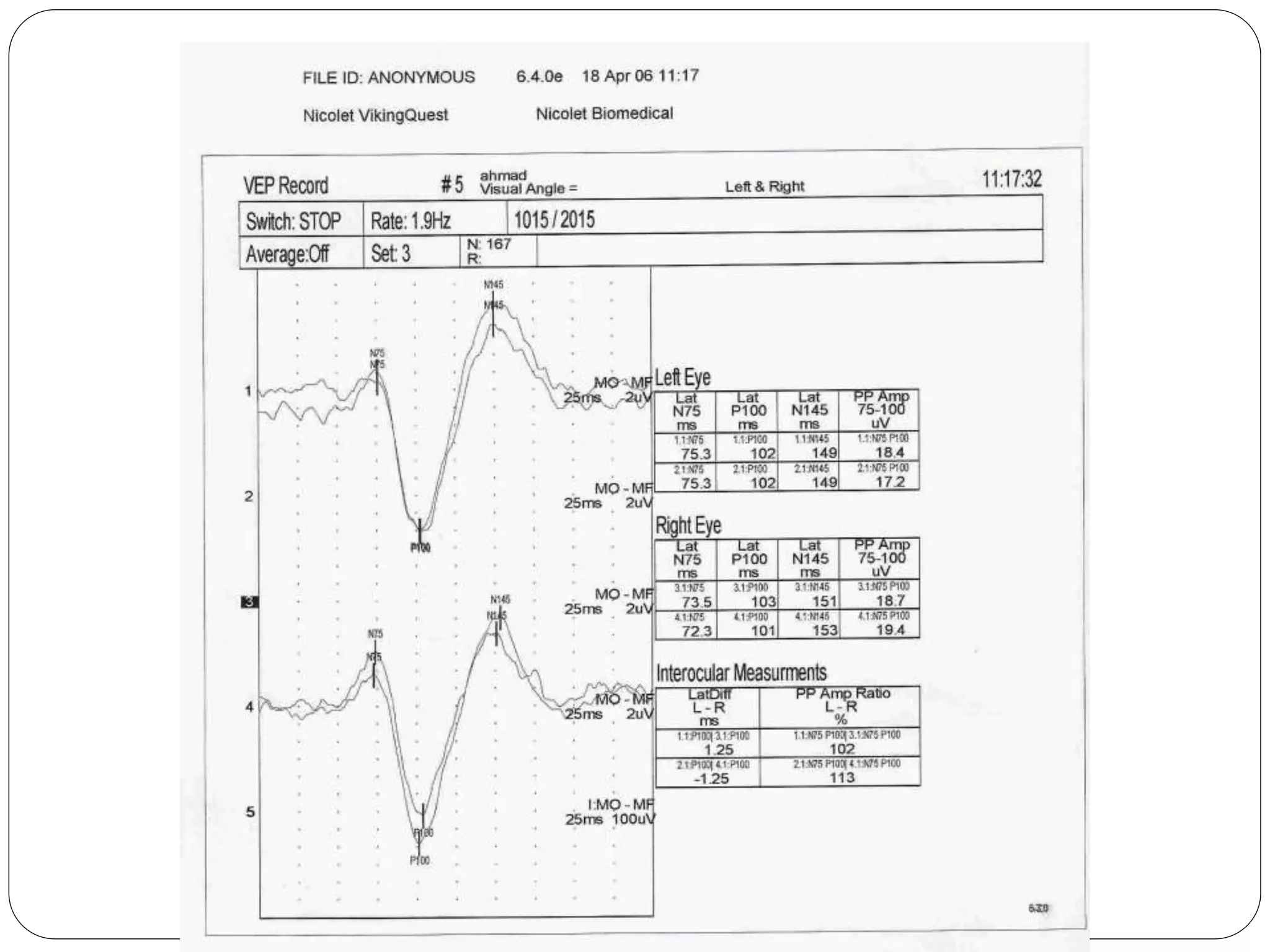
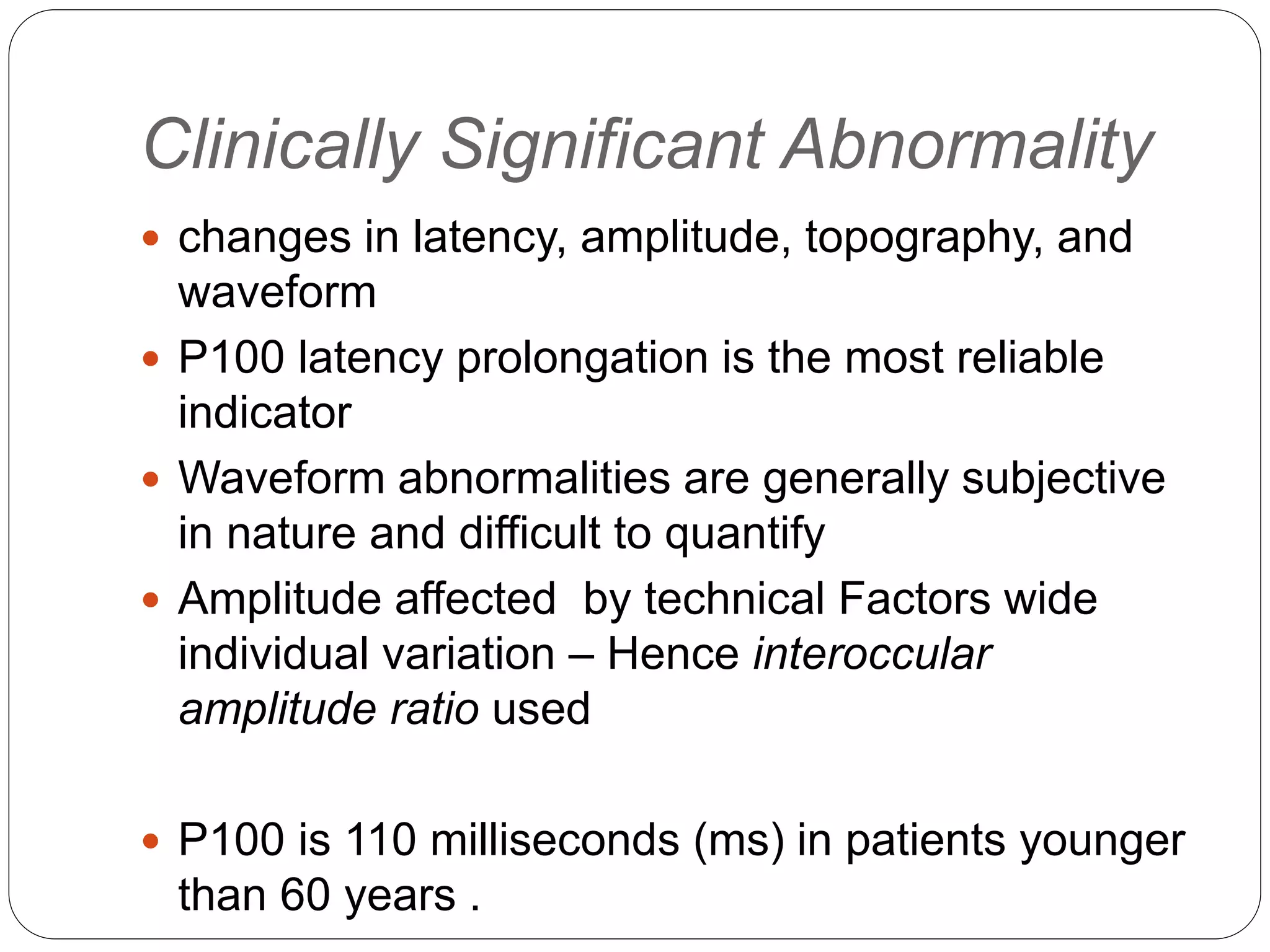
3. VEP involves recording electrical potentials from the visual cortex in response to visual stimuli. Pattern reversal VEP using checkerboard patterns is commonly used to detect lesions of the visual pathway. P100 latency prolongation is the most reliable indicator of abnormality.