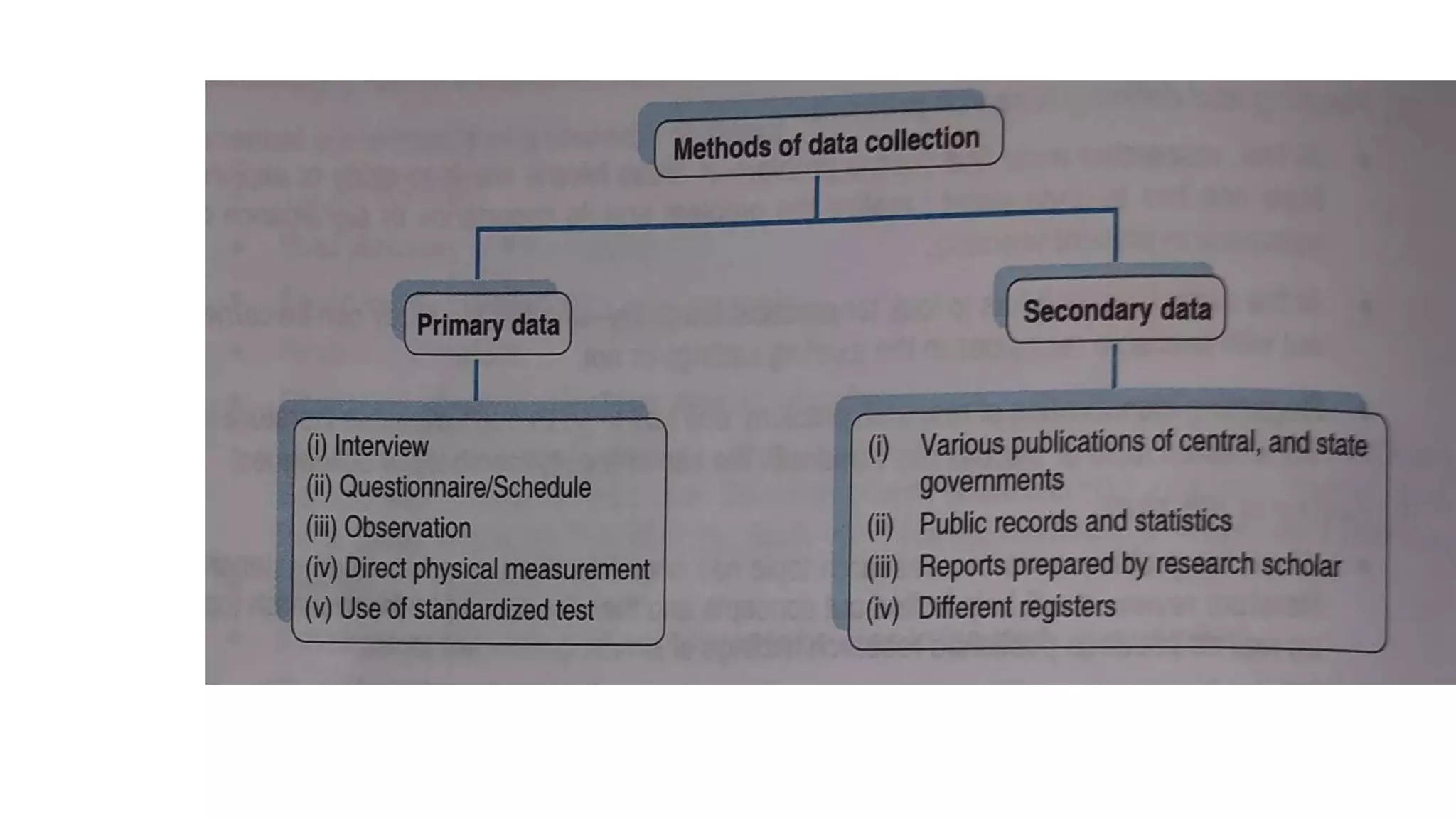
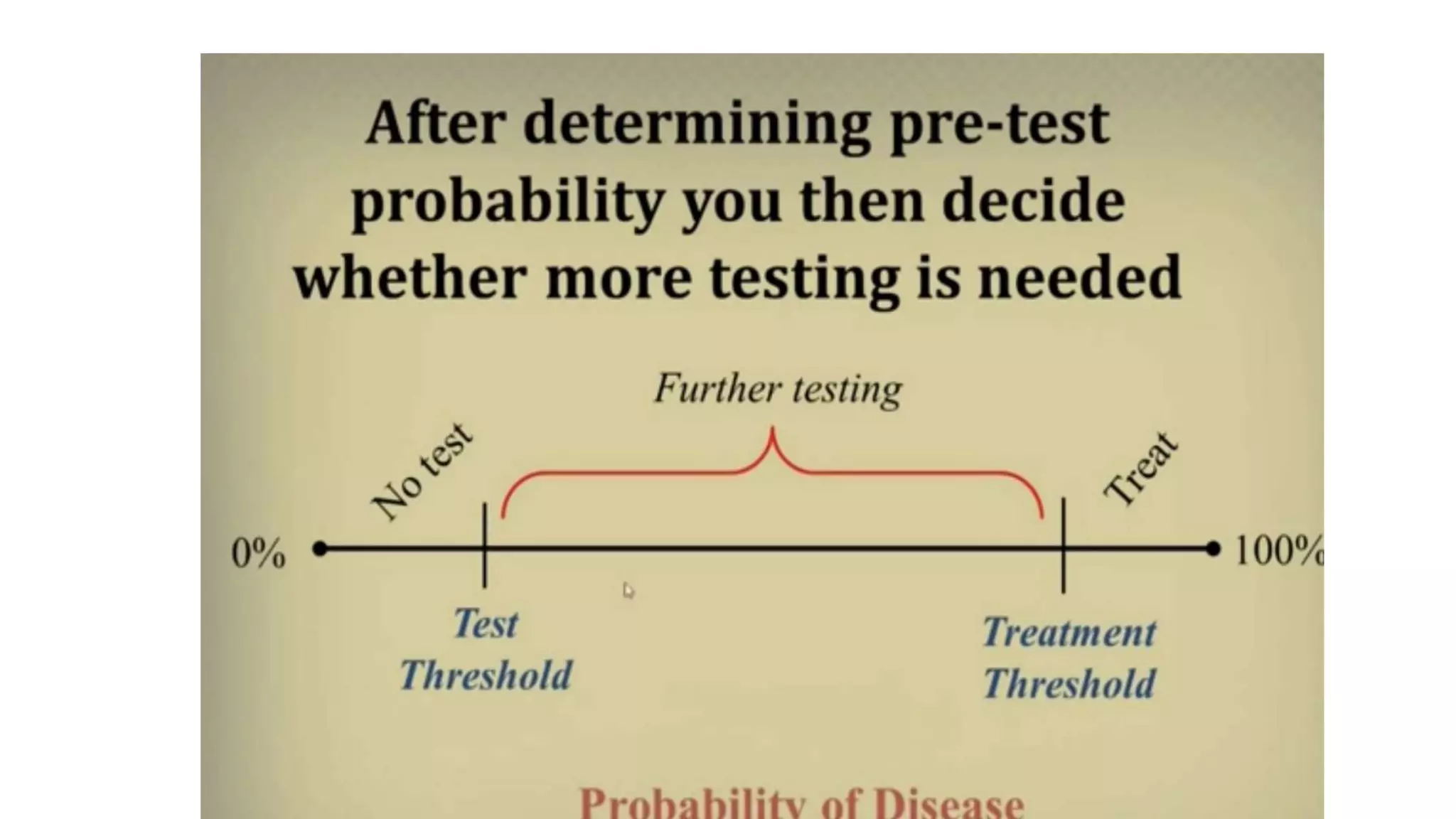
This document provides an overview of the basics of clinical research. It discusses the aims and objectives of clinical research, which should be specific, measurable, attainable, realistic and time-bound. The steps of the research process are outlined, including planning, literature review, developing hypotheses, study design, sampling, data collection, and analysis. Pre-test and post-test probability are explained, which refer to the likelihood of a disease being present before or after a diagnostic test. Factors like a test's sensitivity and specificity should be considered when selecting a test based on whether the goal is to rule a disease in or out.