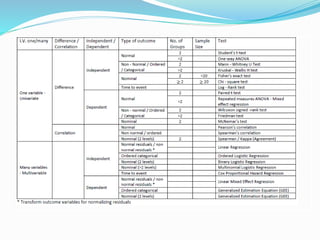
The document discusses sample size estimation and statistical test selection for data analysis in clinical trials. It emphasizes the importance of calculating sample size to achieve study objectives, avoid type I and II errors, and choose appropriate statistical tests based on study design and hypothesis. Various factors affecting sample size, such as effect size, allocation ratio, and the type of outcome, are detailed to guide researchers in their planning and reporting.