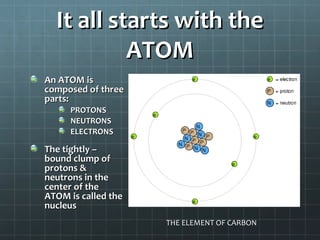
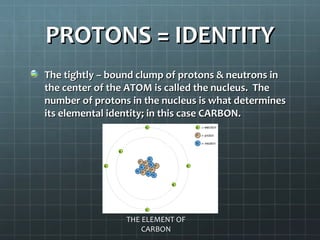
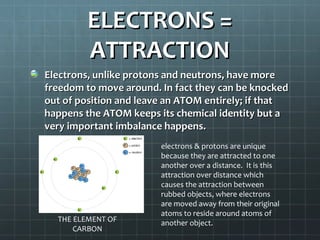
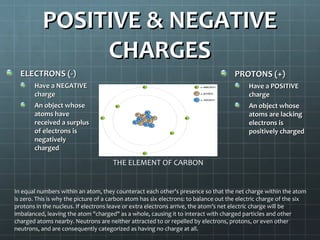
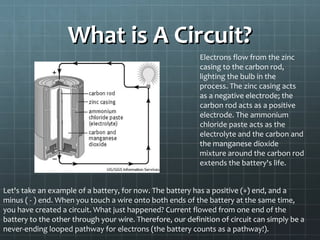
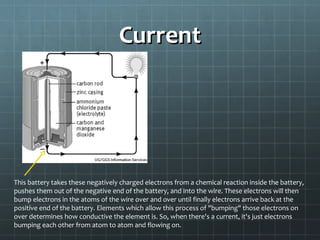
The document discusses basic electrical circuit theory and components. It explains that atoms are composed of protons, neutrons, and electrons. Protons determine elemental identity, neutrons determine mass, and electrons allow for attraction. A circuit is formed when a closed conducting loop allows electrons to flow from the negative terminal of a battery or other power source back to the positive terminal. Key requirements for a circuit include a closed conducting path and connections made only of conductive materials capable of carrying electric charge. Current occurs as electrons bump from atom to atom along a conductor within the circuit.