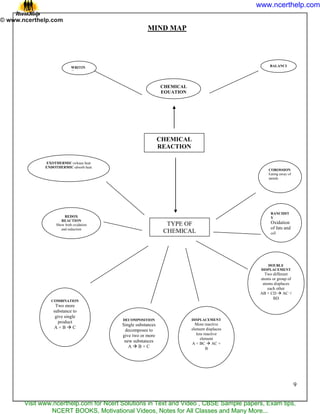
1) A chemical reaction is a process where one or more new substances are formed. Chemical equations represent reactions using symbols for reactants and products.
2) Balanced chemical equations ensure the same number and type of atoms for each element on both sides of the reaction arrow.
3) Common reaction types include combination, decomposition, displacement, and double displacement. Combination reactions form one product from two or more reactants while decomposition reactions break down one reactant into multiple products.