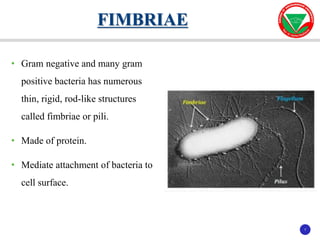
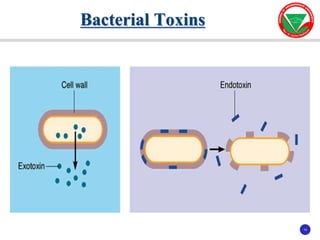
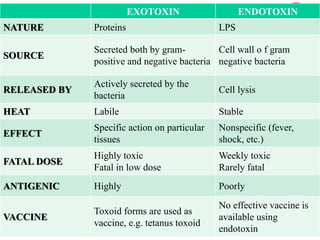
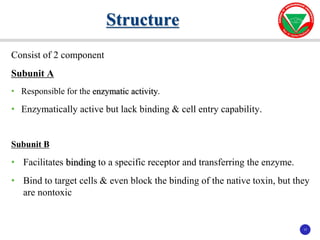
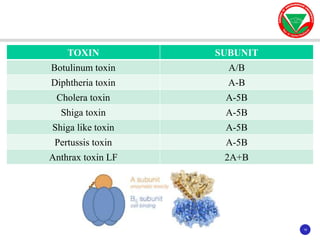
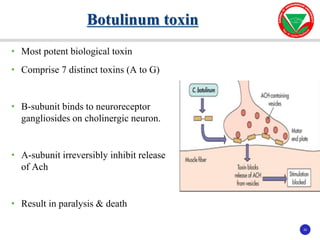
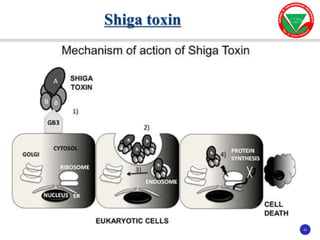
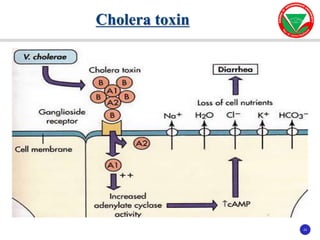
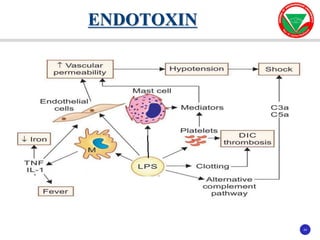
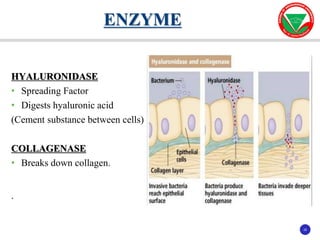
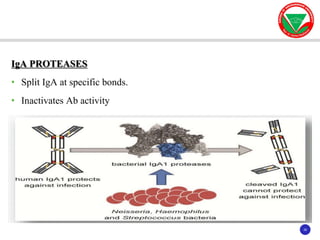
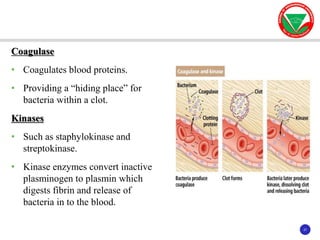
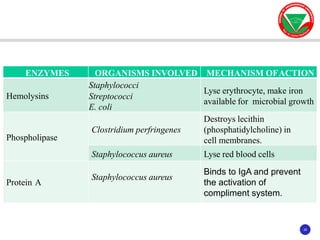
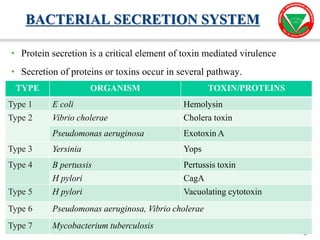
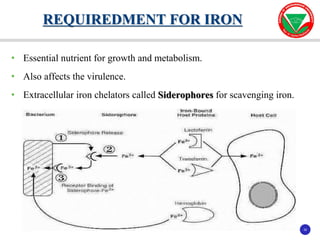
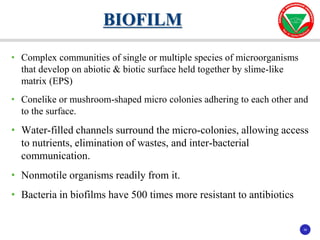
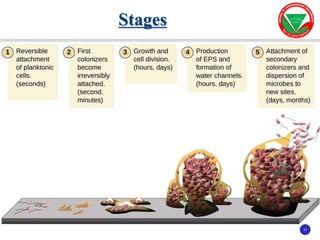
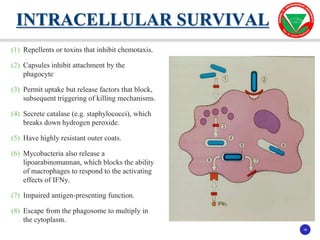
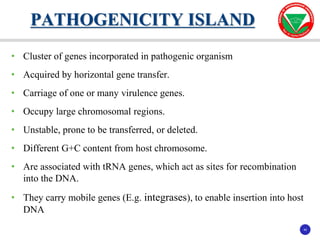
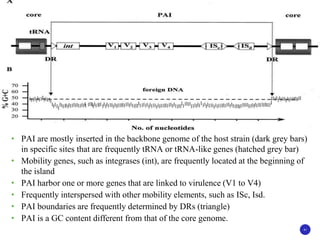
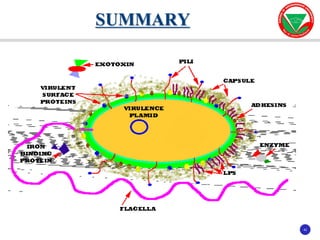
The document discusses bacterial virulence factors. It defines key terms and outlines different types of extracellular and intracellular virulence factors that bacteria use to cause disease, including fimbriae, capsules, toxins, enzymes, secretion systems, and the ability to acquire iron. It describes various virulence factors in detail and explains how they contribute to pathogenicity by mediating adhesion, invasion, intracellular survival, and more.