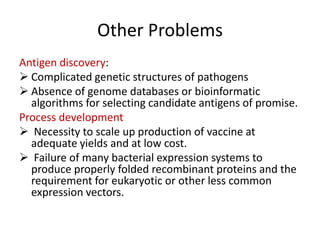
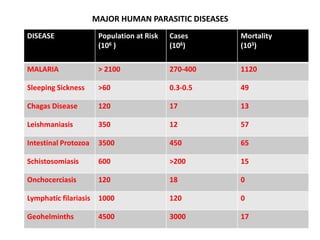
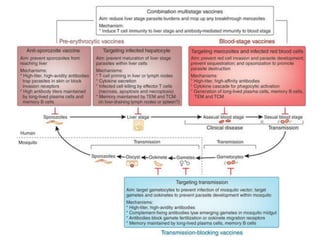
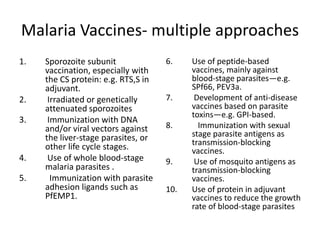
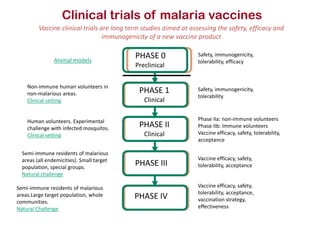
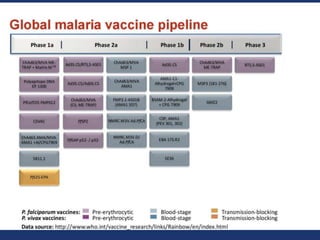
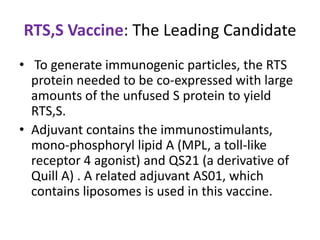
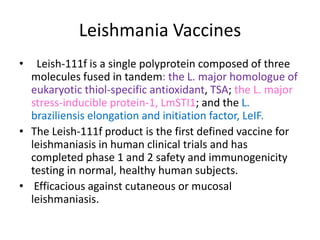
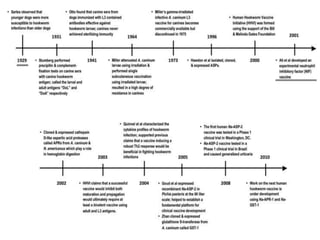
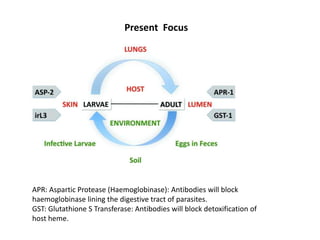
This document discusses human parasite vaccines. It begins by explaining what vaccines do in stimulating the host's protective immune response. Developing effective parasite vaccines faces challenges including not fully understanding the parasite's life cycle and which stages elicit a protective immune response. Effective vaccines must produce long-lasting protection without boosting and be low-cost, stable, and safe. Progress has been limited for parasite vaccines due to parasites' ability to evade the immune system, uncertainty regarding which antigens stimulate protection, and differences between animal models and human immune responses. Major human parasitic diseases discussed include malaria, African sleeping sickness, Chagas disease, leishmaniasis, intestinal protozoa, schistosomiasis, onchocerciasis