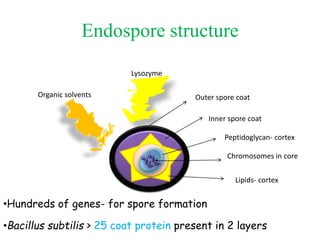
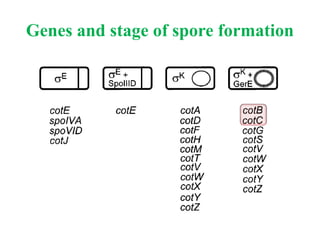
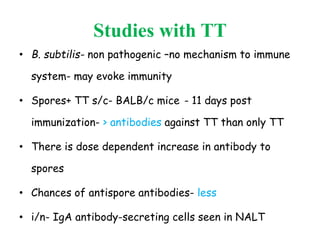
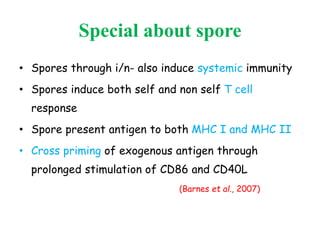
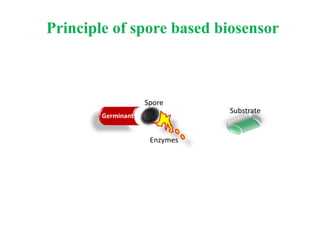
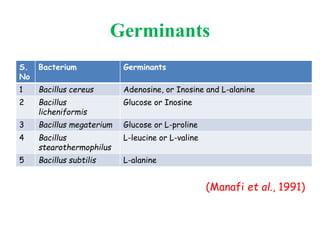
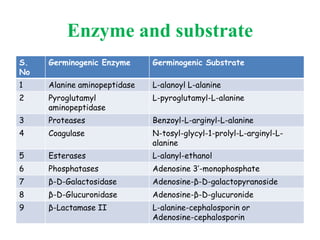
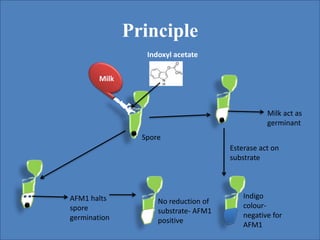
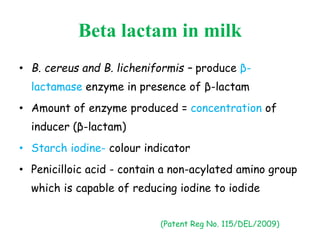
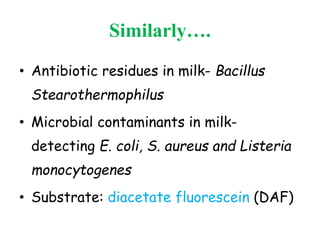
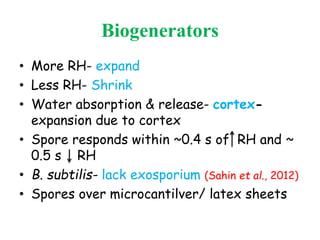
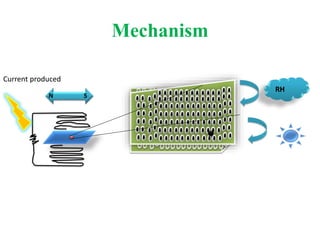
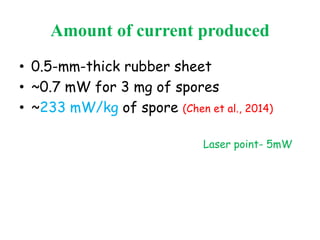
This document discusses bacterial spores and their potential applications. It provides background on spore structure and the genes involved in spore formation. Spores can survive harsh conditions for many years due to their protective coat. This protective property makes spores useful for vaccine delivery, as they can display foreign antigens on their coat and induce immune responses when administered. The document explores using spores that display tetanus and E. coli antigens as oral vaccines. It also discusses using spores as biosensors, where germination in response to certain molecules could generate detectable signals. Finally, the document briefly outlines additional applications of spores in energy generation, self-healing concrete, and cancer treatment.