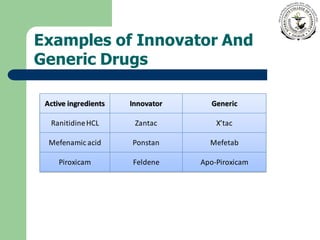
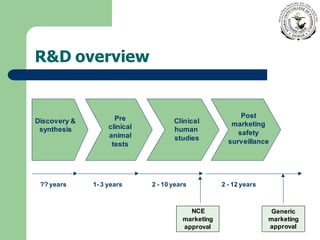
Innovator drugs are the original medications with approved efficacy, safety, and quality, typically protected by a patent for up to 20 years. Generic drugs, made with the same active ingredients, can be marketed after the patent expires and must undergo bioequivalence studies to ensure safety and effectiveness. While there are myths about the inferiority of generics, they are held to the same safety and efficacy standards as innovator drugs, and often come at a lower cost.