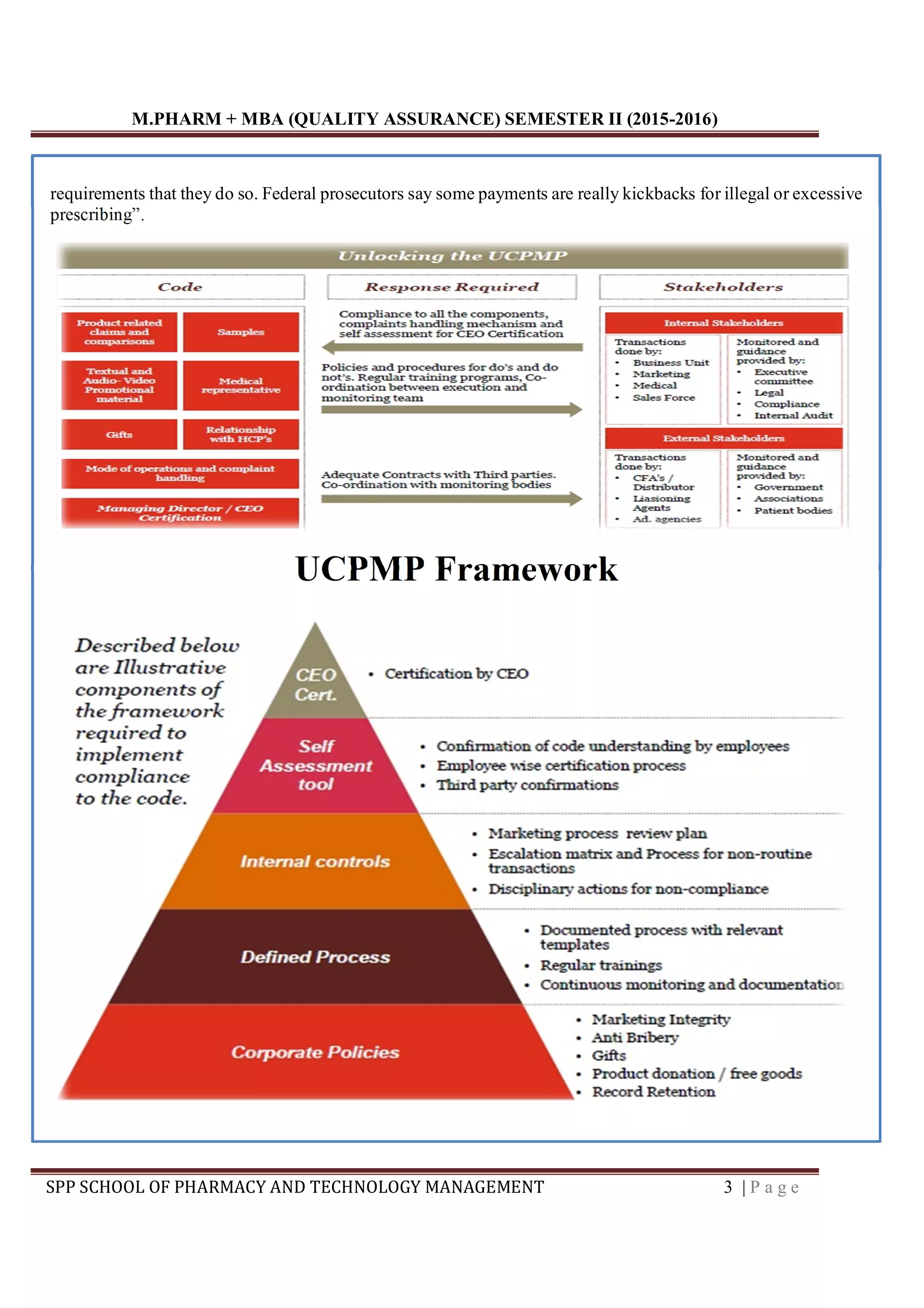
The document discusses pharmaceutical marketing practices in India and the development of a Uniform Code of Pharmaceutical Marketing Practices (UCPMP). It notes that while the UCPMP aims to standardize ethical practices, some remain skeptical of its effectiveness without strict enforcement. Concerns have been raised about the influence of pharmaceutical company promotions on doctor prescribing habits. The UCPMP framework outlines principles for ethical product promotion, prohibiting gifts to influence prescribing, and requiring transparency around expenditures.