This document summarizes key concepts about enzymes and cell biology:
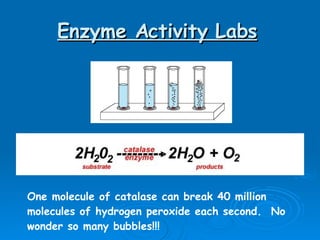
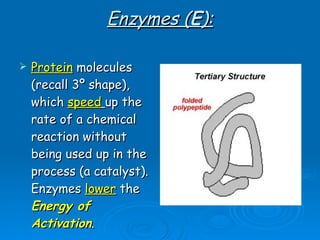
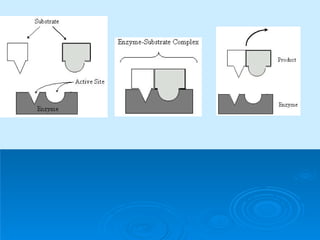
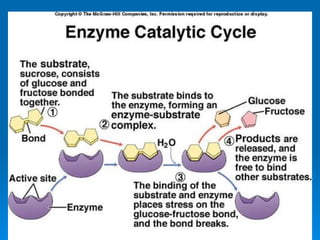
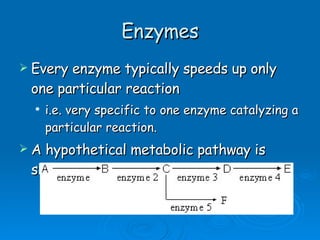
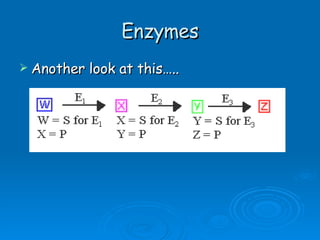
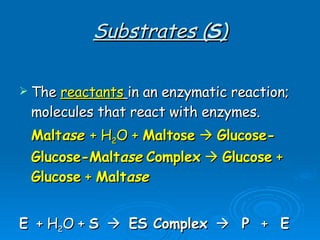
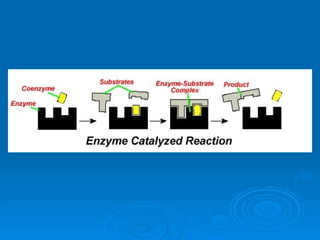
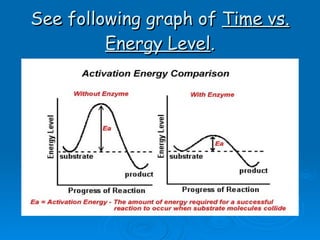
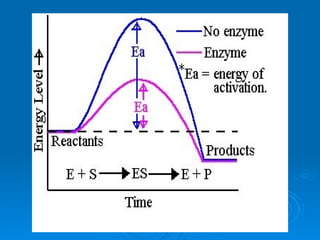
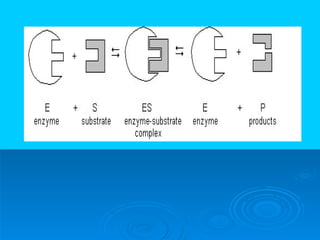
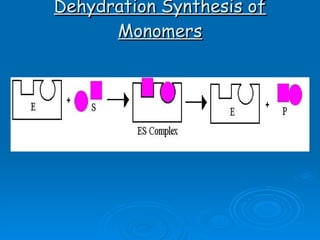
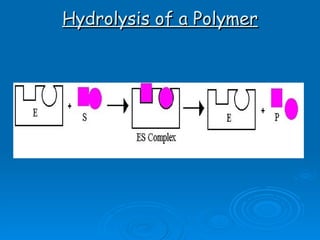
- Enzymes are proteins that speed up biochemical reactions by lowering their activation energy and catalyzing the conversion of substrates to products. They have specific active sites that bind substrates.
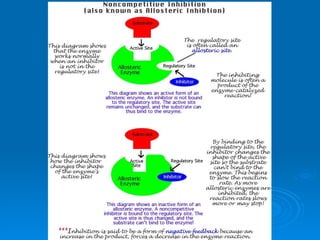
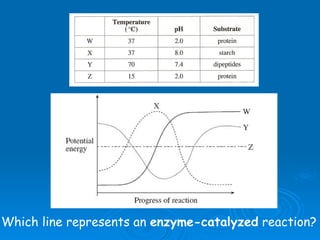
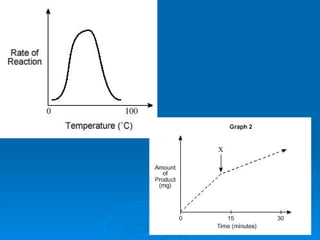
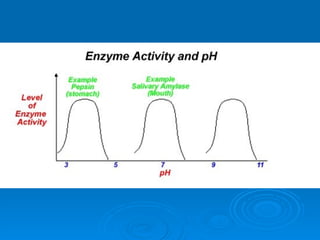
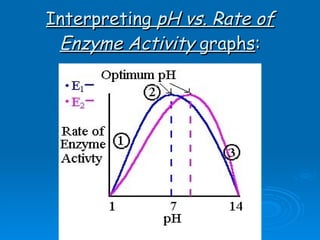
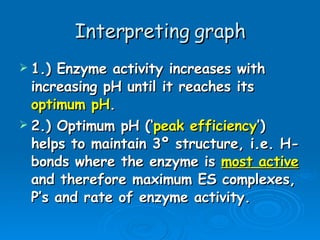
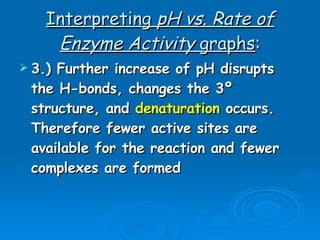
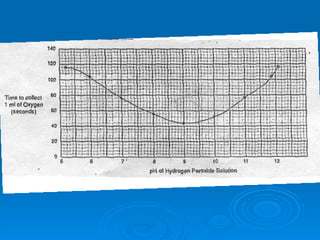
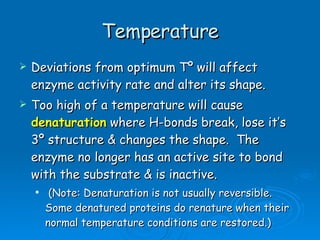
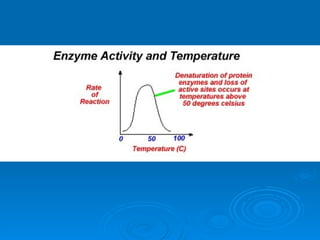
- Factors like pH, temperature, substrate/enzyme concentrations can affect the rate of enzymatic reactions by impacting enzyme structure and activity. Deviations from optimal conditions can cause enzymes to denature.
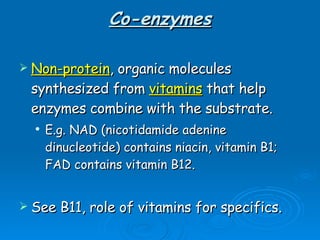
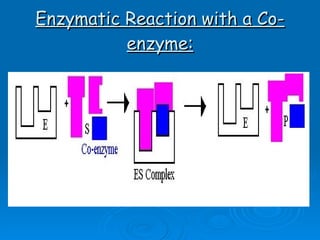
- Coenzymes are non-protein molecules like vitamins that help enzymes catalyze reactions by binding to them.















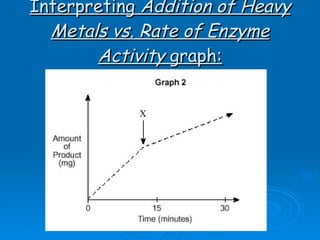
![Factors affecting the rate The following factors affect the rate of enzyme activity and therefore the amount of products produced: pH temperature [substrate] [enzyme] competitive inhibitors non-competitive inhibitors, e.g. heavy metals](https://image.slidesharecdn.com/unit-b11-enzymes-1207890888475167-8/85/B11-Enzymes-36-320.jpg)




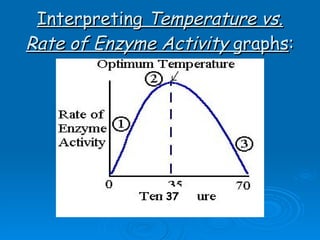
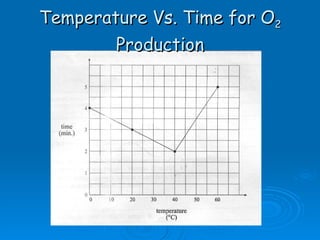
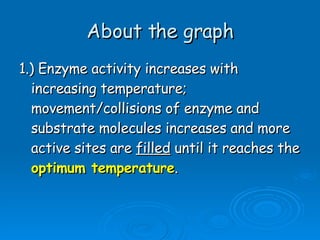
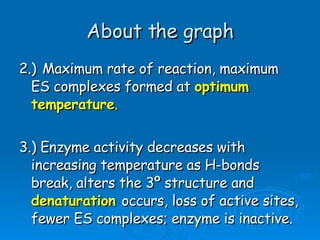
![[Substrate]](https://image.slidesharecdn.com/unit-b11-enzymes-1207890888475167-8/85/B11-Enzymes-52-320.jpg)
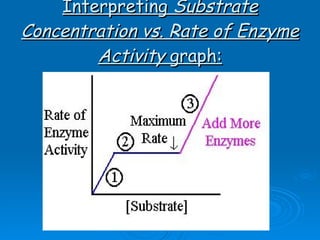
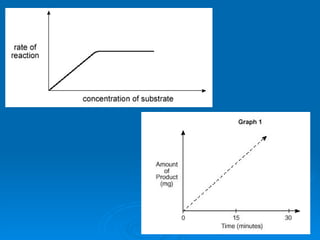
![About the Substrate graph 1.) Enzyme activity increases as [substrate] increases and reaction rate increases to a point. 2.) Enzyme activity slows down and levels off reaching the maximum rate . The substrate exceeds the number of enzymes and active sites are all occupied . E.g. All maltase activity sites are in use. Note: Adding more enzymes (see ‘3’ in graph above) will further increase the rate of enzyme activity as there are more available enzymes and active sites for the substrate.](https://image.slidesharecdn.com/unit-b11-enzymes-1207890888475167-8/85/B11-Enzymes-54-320.jpg)
![[Enzyme]](https://image.slidesharecdn.com/unit-b11-enzymes-1207890888475167-8/85/B11-Enzymes-55-320.jpg)
![About the Enzyme graph Reaction rate increases as [enzyme] increases (to the same increasing [substrate]). The same amount of products will be produced.](https://image.slidesharecdn.com/unit-b11-enzymes-1207890888475167-8/85/B11-Enzymes-56-320.jpg)
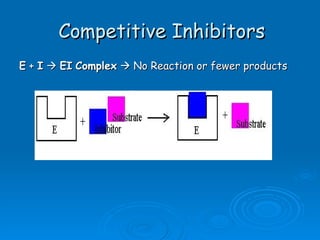
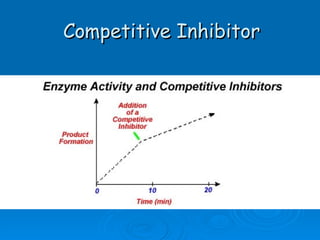
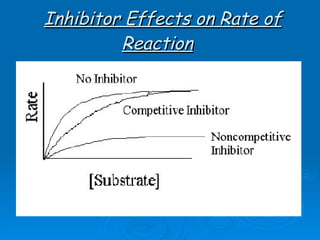
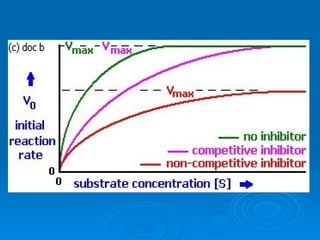
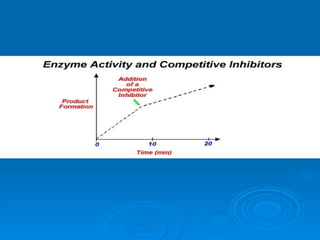
![Competitive Inhibitor Chemicals that have the same shape as the substrate and will compete for the active site. Enzyme cannot react with the “look-a-like”. This effectively reduces the [of available enzyme] and inhibits/decreases the reaction. The effect of competitive inhibitors can be overcome by increasing the [substrate].](https://image.slidesharecdn.com/unit-b11-enzymes-1207890888475167-8/85/B11-Enzymes-57-320.jpg)