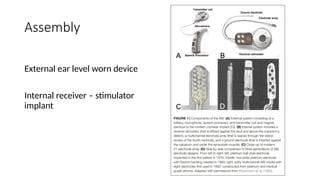
The document provides an overview of auditory brainstem implants (ABI), a neuroprosthetic device designed to restore hearing in patients ineligible for cochlear implants due to various cochlear and nerve pathologies. It details the history, patient criteria, surgical considerations, and results, indicating that 82% of users can perceive sound post-operation, with significant improvements in speech understanding when combined with lip-reading. Recent advancements in optogenetics and conformable electrode designs are also discussed, highlighting potential future enhancements in ABI technologies.