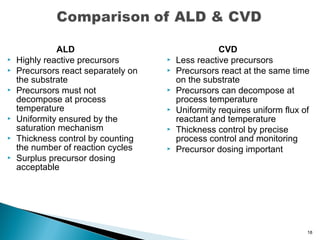
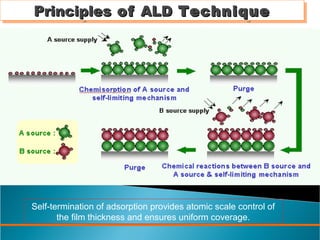
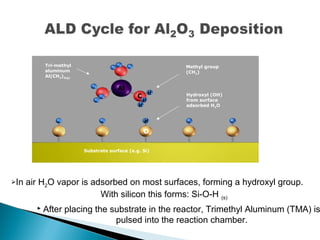
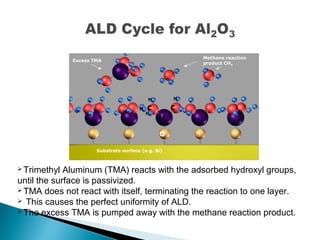
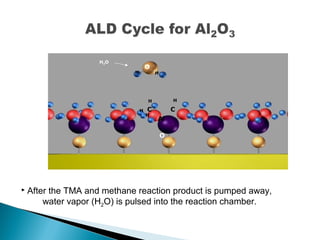
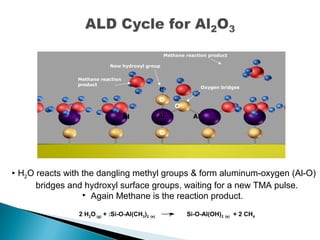
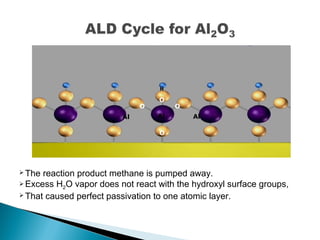
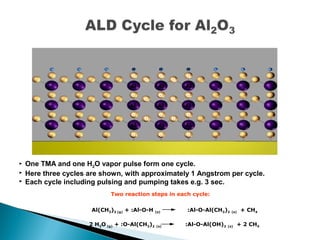
ALD is a thin film deposition technique based on self-terminating surface reactions of gas precursors. It involves alternating exposure of a substrate to different precursors separated by purge steps, resulting in one atomic layer of film growth per cycle. ALD provides highly conformal and uniform coatings with atomic-level thickness control due to its self-limiting growth mechanism. It is widely used for depositing oxides, nitrides and some metals in applications such as semiconductors, coatings, MEMS and solar cells.





![16
The Verano 5500™
A 300-mm ALD system by
Aviza Technology, Inc [2].
Process Temperature [1]
[1] [1]
11
"Technology Backgrounder: Atomic Layer Deposition," IC Knowledge LLC, 24 April 06. <"Technology Backgrounder: Atomic Layer Deposition," IC Knowledge LLC, 24 April 06. <
www.icknowledge.com/misc_technology/Atomic%20Layer%20Deposition%20Briefing.pdfwww.icknowledge.com/misc_technology/Atomic%20Layer%20Deposition%20Briefing.pdf>>
22
”Atomic Layer Deposition," Aviza Technology. 26 April 06. <”Atomic Layer Deposition," Aviza Technology. 26 April 06. <
http://www.avizatechnology.com/products/verano.shtmlhttp://www.avizatechnology.com/products/verano.shtml>.>.](https://image.slidesharecdn.com/mukhtarald-140717070420-phpapp01/85/Atomic-layer-Deposition-_Mukhtar-Hussain-awan-16-320.jpg)