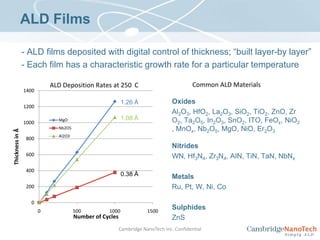
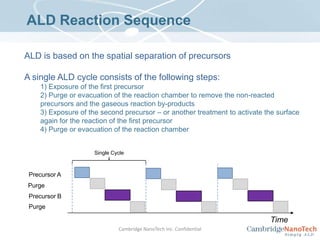
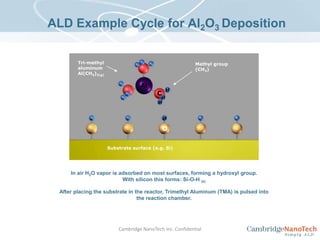
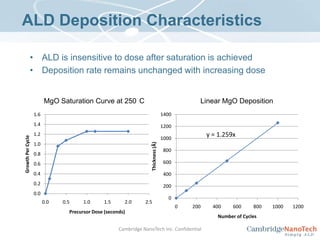
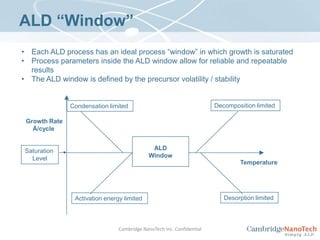
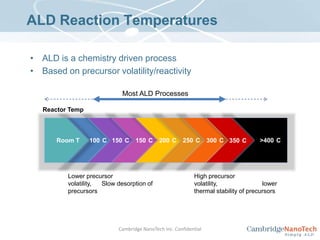
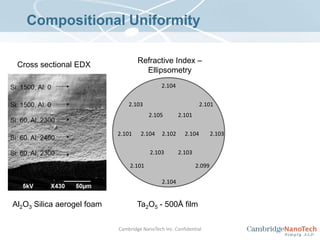
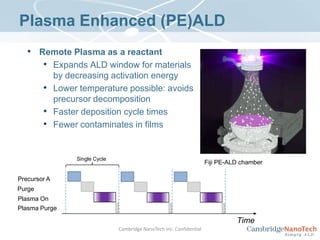
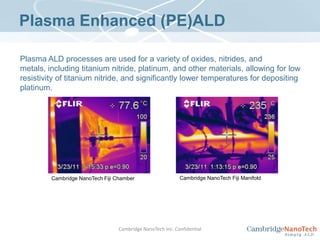
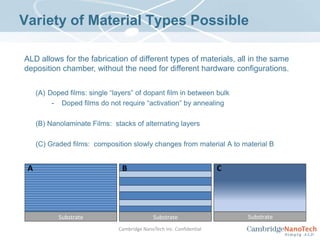
This document discusses atomic layer deposition (ALD) applications, films, deposition characteristics, and reaction sequences. It summarizes that ALD allows for precise, conformal deposition of various oxide, nitride, and metal thin films. ALD is well-suited for applications requiring high aspect ratio coatings, compositional uniformity, and compatibility with sensitive substrates.