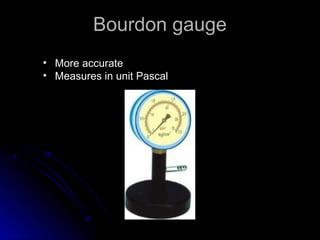
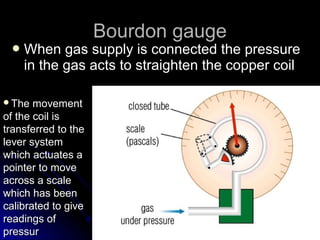
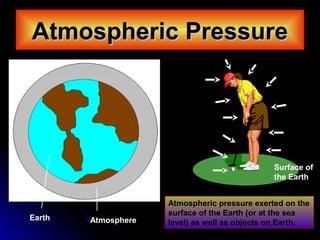
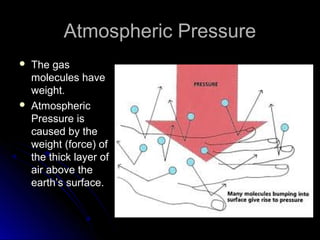
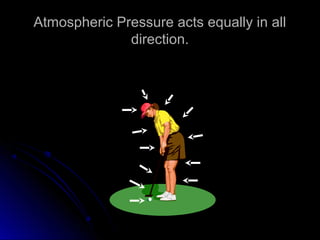
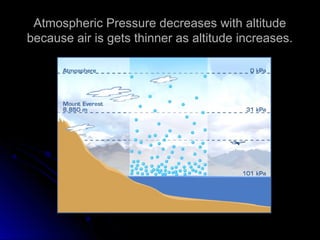
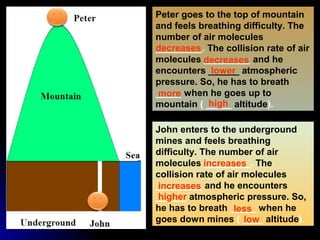
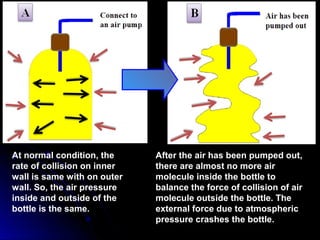
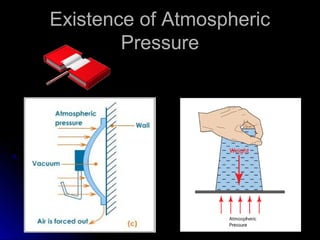
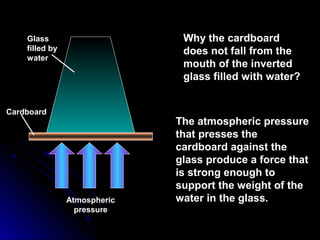
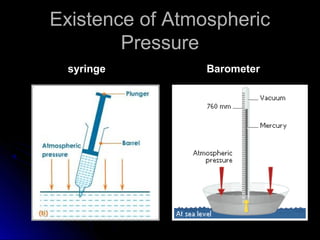
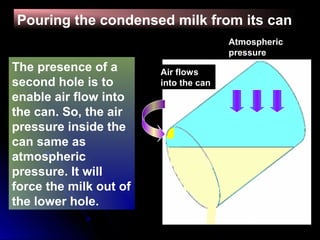
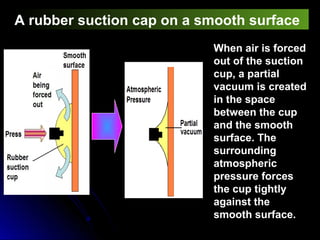
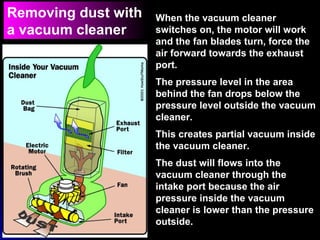
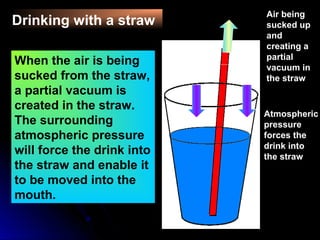
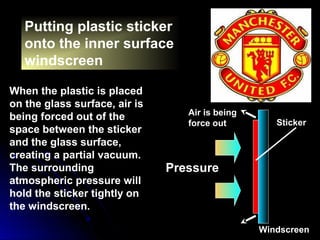
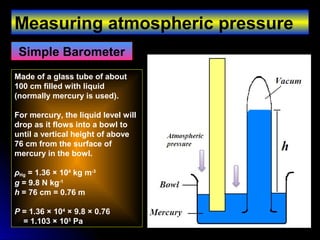
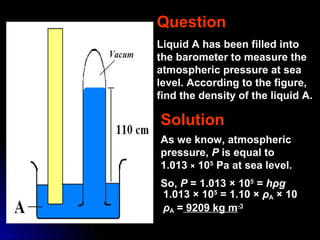
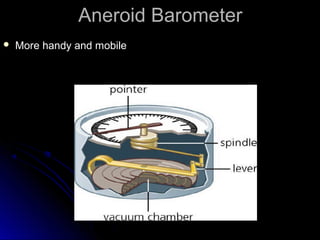
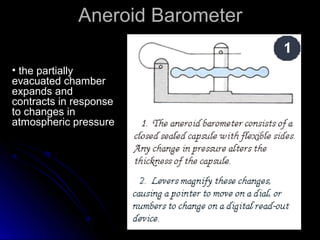
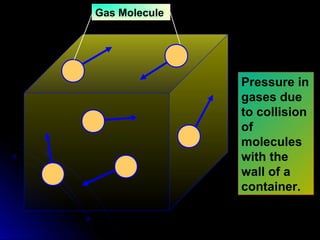
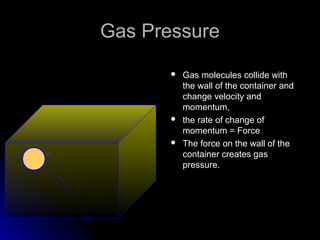
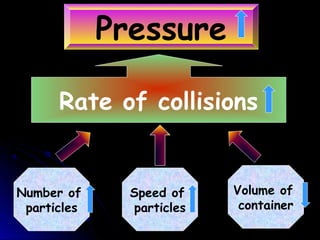
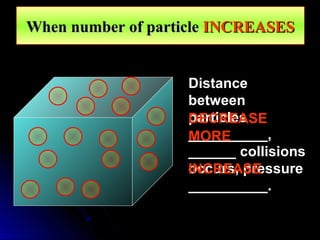
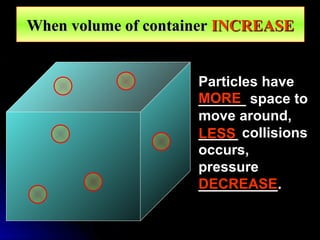
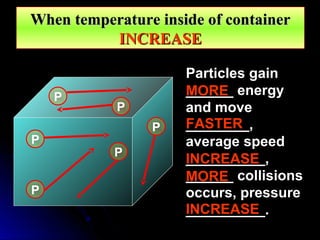
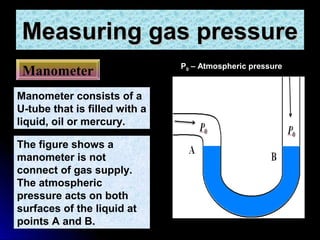
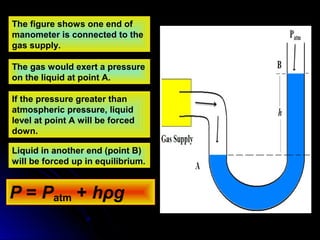
The document discusses atmospheric pressure and how it is caused by the weight of air above the Earth's surface. It explains that atmospheric pressure decreases with increasing altitude as there is less air above. Instruments like mercury barometers, aneroid barometers, and manometers can be used to measure atmospheric pressure. Gas pressure inside a container is also explained using kinetic molecular theory, where gas molecule collisions with the container walls exert pressure.













![Question
A mercury manometer with one end attached
to a gas supply measures a difference in the
level of mercury of 32 cm as in figure.
Calculate the pressure of the gas supply in
(a) cmHg (b) Pascal
[ Patm = 76 cmHg; g = 10 Nkg-1
;
ρmercury = 1.36 × 104
kgm-3
]
Solution
(a) Pressure = Atmospheric pressure +
pressure due to mercury column
= 76 cmHg + 32 cmHg
= 108 cmHg
(b) Pressure of gas supply = hρg= 108 × 10-2
× 1.36 × 104
× 10
= 1.46 × 105
Pa](https://image.slidesharecdn.com/atmosphericngaspressure-130724234958-phpapp02/85/Atmospheric-n-gas-pressure-42-320.jpg)
![Question
A mercury manometer with one end attached
to a gas supply measures a difference in the
level of mercury of 10 cm as in figure.
Calculate the pressure of the gas supply in
(a) cmHg (b) Pascal
[ Patm = 76 cmHg; g = 10 Nkg-1
;
ρmercury = 1.36 × 104
kgm-3
]
Solution
(a) Pgas = PHg + Patm
= 10 cmHg + 76 cmHg
= 86 cmHg
(b) Pressure of gas supply = hρg
= 86 × 10-2
× 1.36 × 104
× 10
= 1.1696 × 105
Pa](https://image.slidesharecdn.com/atmosphericngaspressure-130724234958-phpapp02/85/Atmospheric-n-gas-pressure-43-320.jpg)