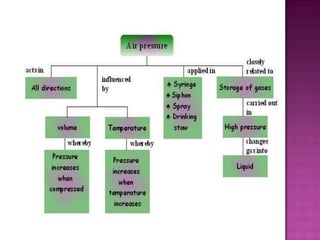
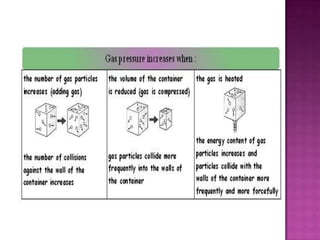
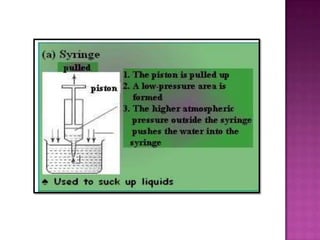
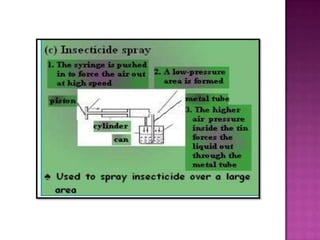
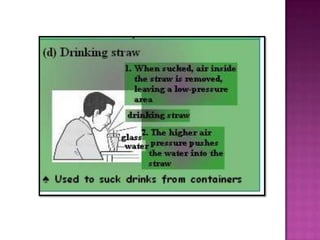
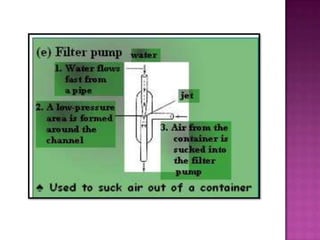
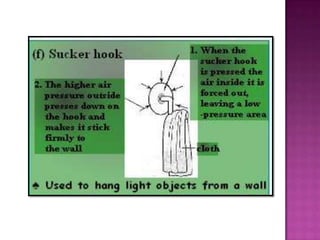
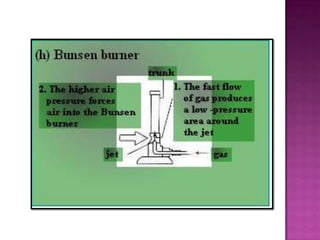
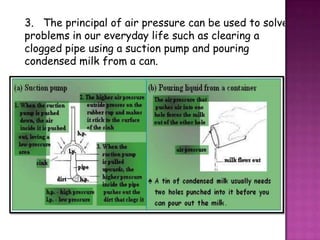
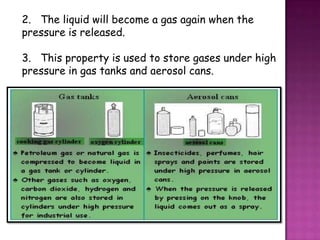
Air pressure is caused by air particles constantly moving and colliding with surfaces. It presses down on everything from above and its strength decreases with increasing altitude as there is less air. Many tools like syringes and sprayers use air pressure principles in their functions. Safety measures must be followed when working with pressurized gases.