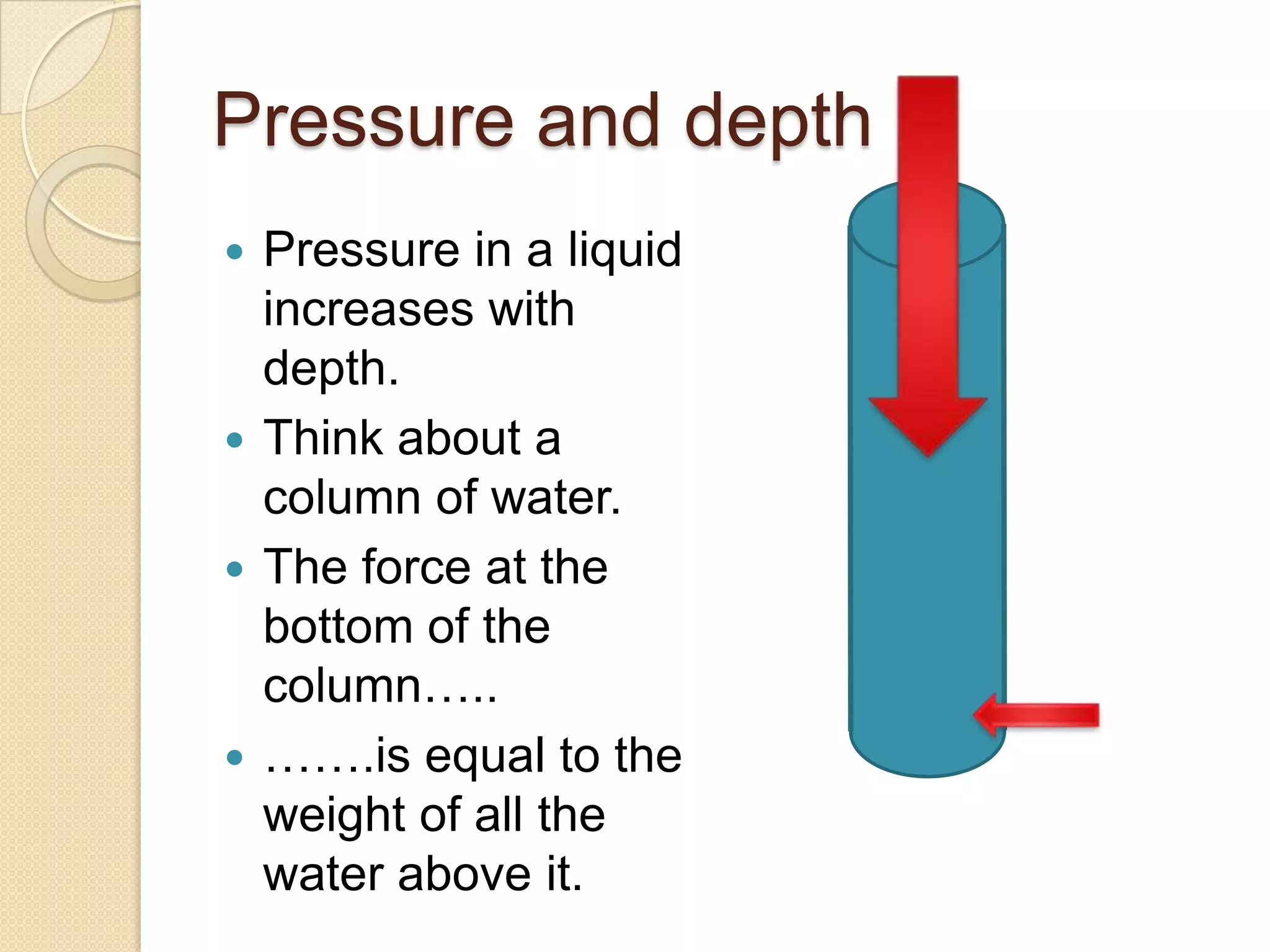
Liquids and gases exert pressure equally in all directions. Pressure increases with depth in liquids and gases due to the weight of the fluid above pressing down. Magdeburg hemispheres demonstrate that air pressure keeps spheres stuck together when the air between them is removed. The formula for calculating fluid pressure is Pressure = Density x Gravity x Height, where increasing height results in increasing pressure.