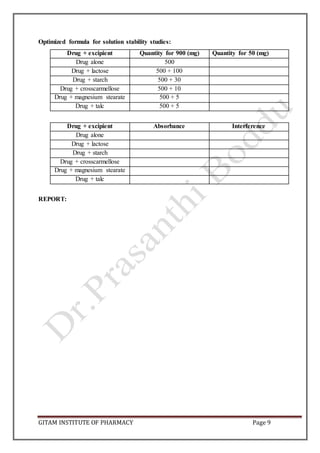
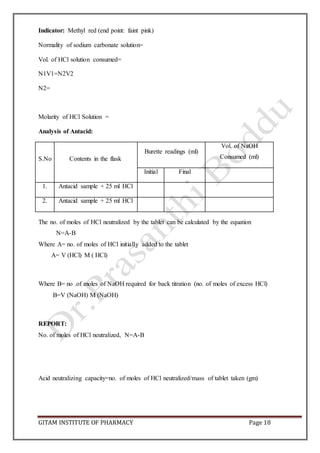
This document outlines preformulation studies conducted on the drug metronidazole, including characterization of its physical properties, solubility, stability, and compatibility with excipients. Key aspects that were evaluated include particle size, bulk density, angle of repose, pH, partition coefficient, stability under various conditions like temperature, humidity and light. Drug-excipient compatibility was also studied by storing mixtures at elevated temperature and observing for physical or chemical changes. The goal of these studies is to understand the drug's characteristics and behavior to aid in rational formulation design and selection of appropriate excipients and storage conditions.


![GITAM INSTITUTE OF PHARMACY Page 4
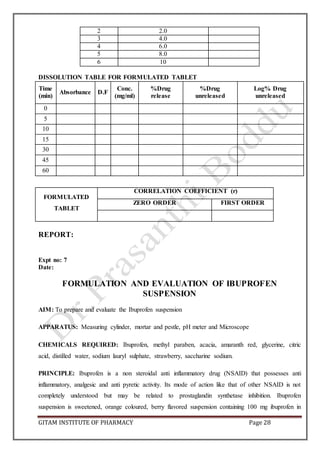
BULK DENSITY: Apparent bulk density (ρb) was determined by placing the granules into a
graduated cylinder and measuring the volume (Vb) and weight (M) “as it is”.
pb = M/Vb
Weight of sample =
Volume of sample =
Bulk density =
TAPPED DENSITY: The measuring cylinder containing a known mass of granules was tapped for
100 times using a bulk density apparatus. The minimum volume (Vt) occupied in the cylinder and the
weight (M) of the granules was measured. The tapped density (pt) was calculated using the formula.
pb = M/Vb
Tapped volume =
Tapped density =
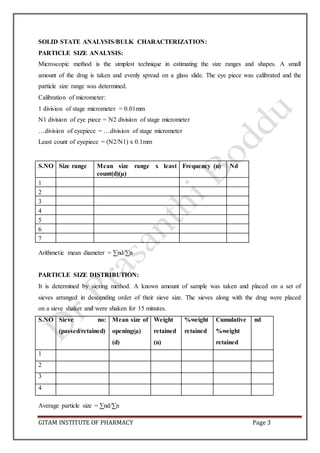
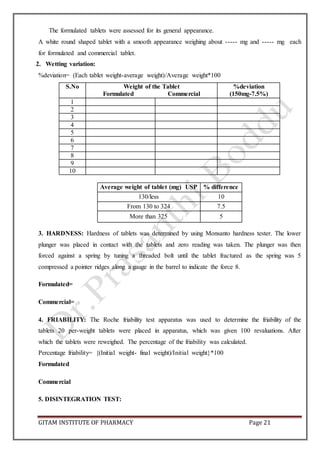
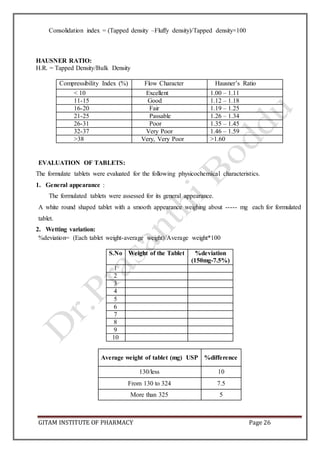
CARR’S CONSOLIDATION INDEX: It is the measure of potential strength that a power could
build up in its arch in a hopper and also the ease with which such an arch could be broken.
Compressibility index of the granules was determined by using the formula.
CI (%) = [(pt-pt/pt)] x 100
Carr’s index (%) Type of flow
5-15 Excellent
12-16 Good
18-21 Fair to passable
23-35 Poor
33-38 Very poor
>40 Extremely poor
Bulk density =
Tapped density =
CI =
HAUSENER’S RATIO: It is the measure of the flow property of the drug.
Hausener’s ratio = pt/pb](https://image.slidesharecdn.com/aptlabmanual-211019063126/85/Apt-lab-manual-4-320.jpg)





















![GITAM INSTITUTE OF PHARMACY Page 36
CHEMICALS: Diclofenac sodium, HPMC, glycerin, distilled water.
APPARATUS: Volumetric flask, digital pH meter, UV – spectrophotometer.
PRINCIPLE:
For topical treatment of dermatological disease as well as skin care, a wide variety of vehicles
ranging from solids to semisolids and liquid preparation is available to clinicians and patients. Within
the major group of semisolids preparations, the use of transparent gels has expended both in
cosmetics and in pharmaceutical preparations. A gel is colloid that is typically 99% weight liquid,
which is immobilized by surface tension between it and a macromolecule network of fibers built
from a small amount of a gelating substance present. Topical drug is administration is localized drug
delivery system anywhere in the body through ophthalmic, rectal, vaginal and skin as topical route.
Skin is the one of the most readily accessible organs of human body for topical administration and
main route of topical drug delivery system. Number of medicated products is applied to the skin or
mucous membrane that either enhances or restore of a fundamental function of a skin or
pharmacologically alter an action in the underline tissue.
DRUG PROFILE:
Molecular structure:
Molecular formula: C14H11Cl2NO2
Molecular weight: 328.1
Chemical name: Sodium 2[2,6-dichlorophenyl)-amino] phenyl acetate.
Category: Analgesic, anti inflammatory
Dose: Orally or by intra muscular injection 25-75mg
Description: A white to slightly yellowish crystalline powder
Solubility: Freely soluble in methanol, soluble in ethanol, sparingly
soluble in water, partially insoluble in ether and chloroform.
PROCEDURE:
PREPARATION OF STANDARD STOCK SOLUTION: A 1mg/ml solution of drug was prepared
by dissolving 50mg diclofenac in 50ml of pH 6.8 phosphate buffer.
From this stock solution 2, 4, 6, 8, 10µg/ml solutions were prepared by diluting with pH 6.8
phosphate buffer. The absorbance was measured at 285nm and a graph was plotted between
concentration Vs absorbance.
PREPARATION OF GEL:
1g of diclofenac sodium was weighed and dissolved in 15ml of glycerin with the help of mild
heat (solution A). Weighed HPMC was added to the 75ml of distilled water and stirred until](https://image.slidesharecdn.com/aptlabmanual-211019063126/85/Apt-lab-manual-36-320.jpg)


