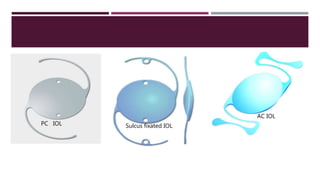
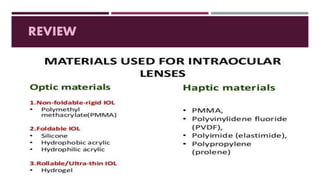
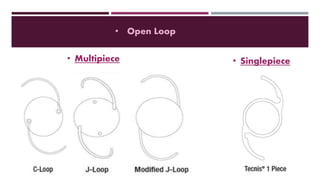
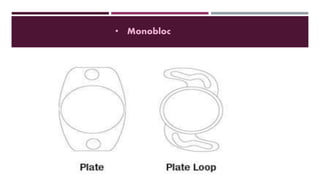
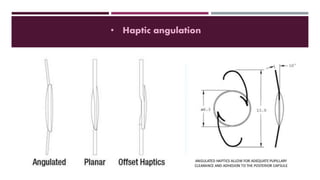
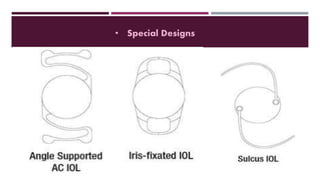
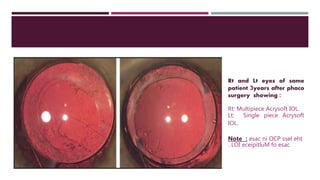
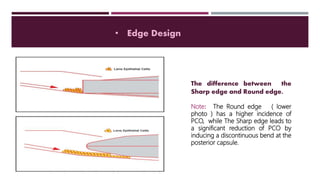
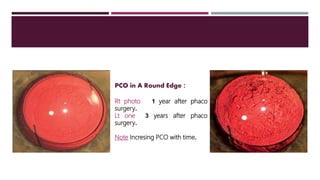
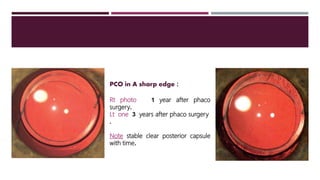
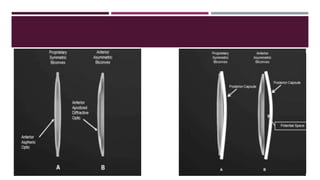
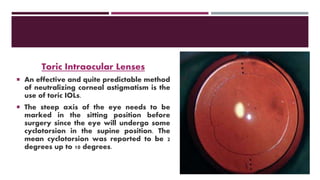
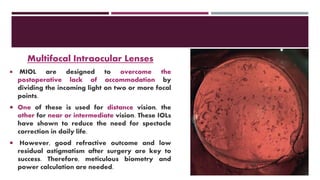
This document discusses different types of intraocular lenses (IOLs) used in cataract surgery. It covers various factors that influence IOL choice such as material, design, and patient needs. The main materials discussed are polymethylmethacrylate, hydrophobic acrylic, hydrophilic acrylic, and silicone. IOL designs include multifocal, toric, and aspherical lenses. Special considerations are mentioned for choosing IOLs in cases of incomplete capsule support, high myopia, or uveitis. The document provides details on characteristics and indications for different IOL materials and designs.