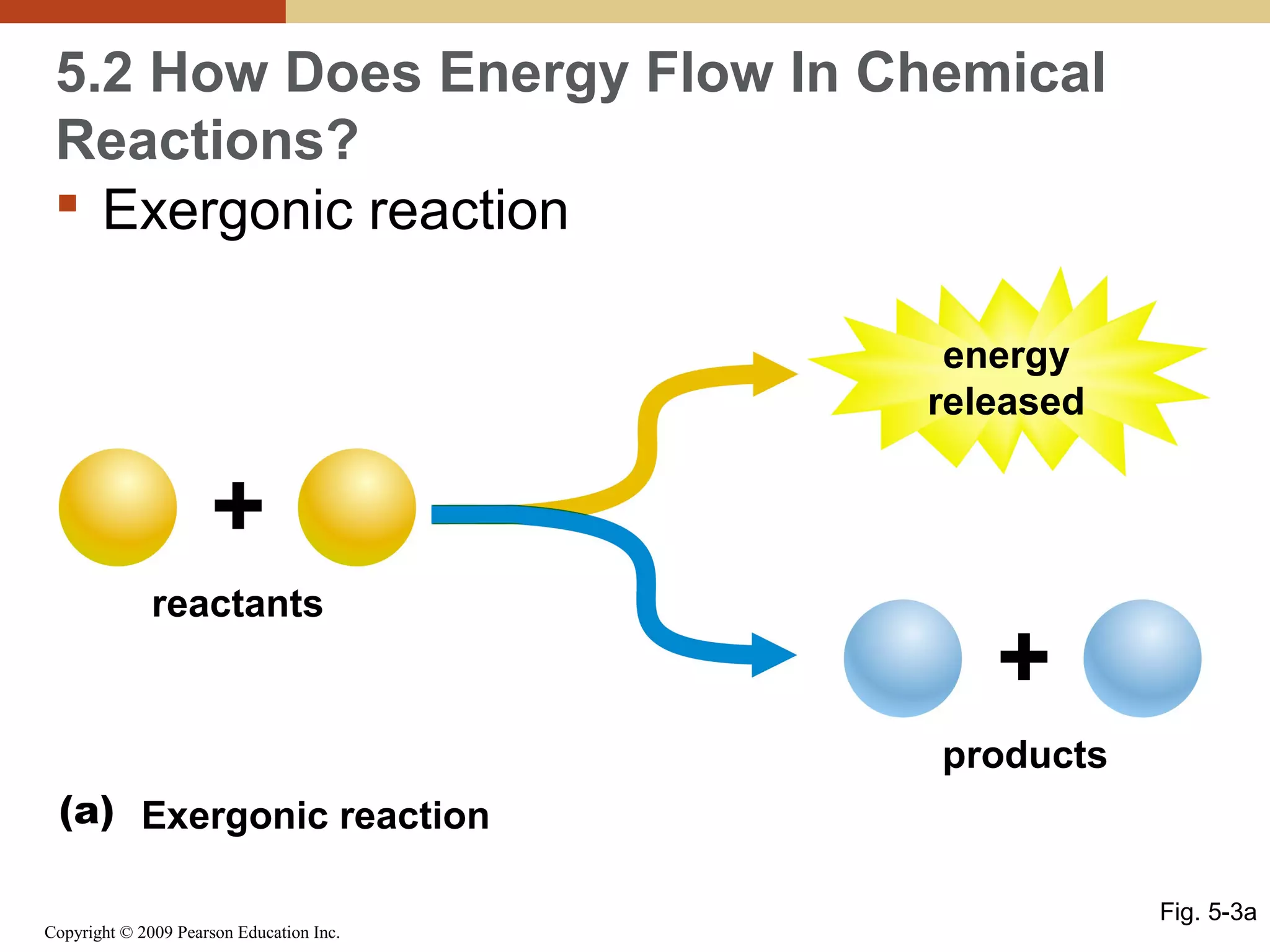
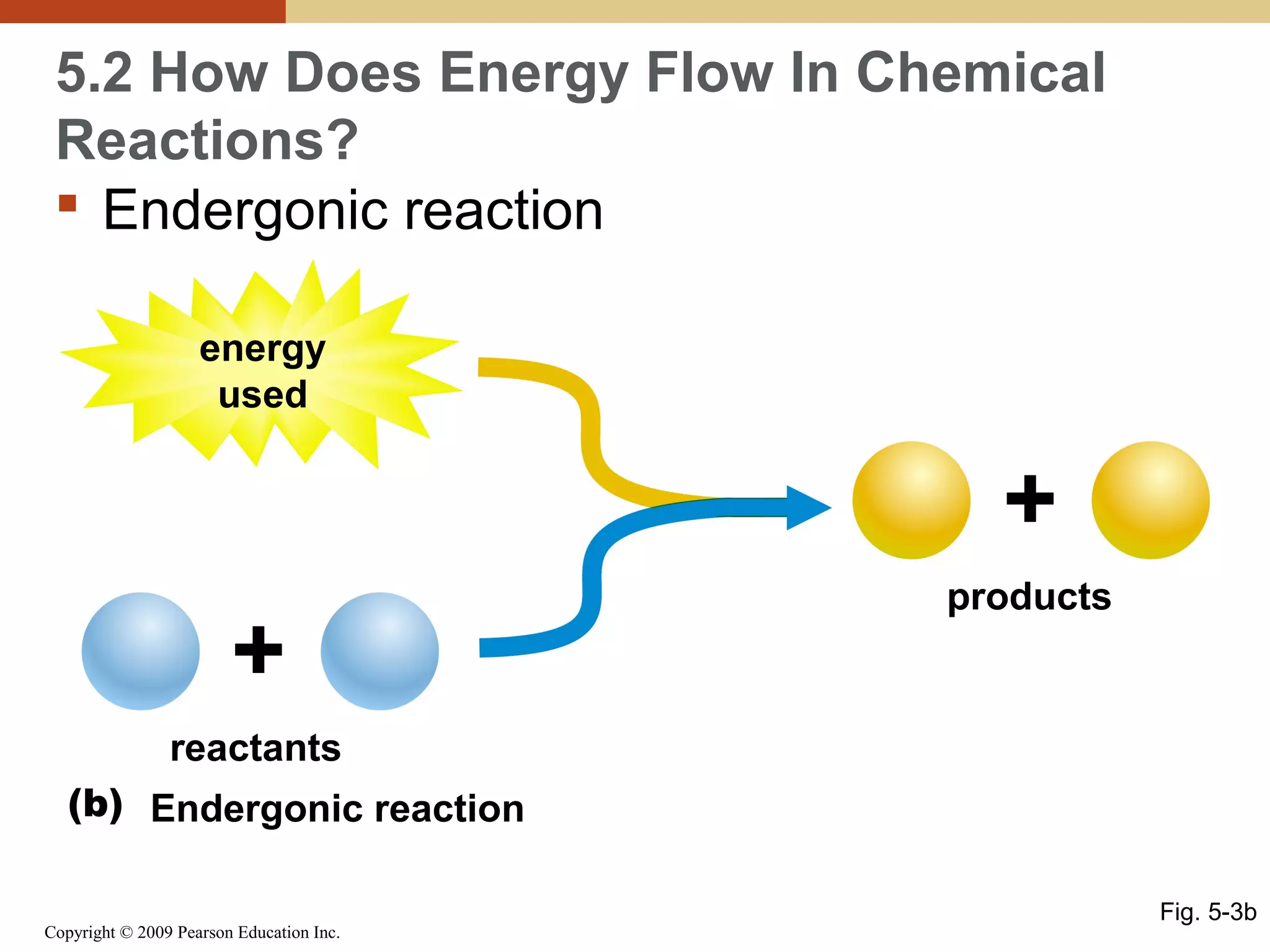
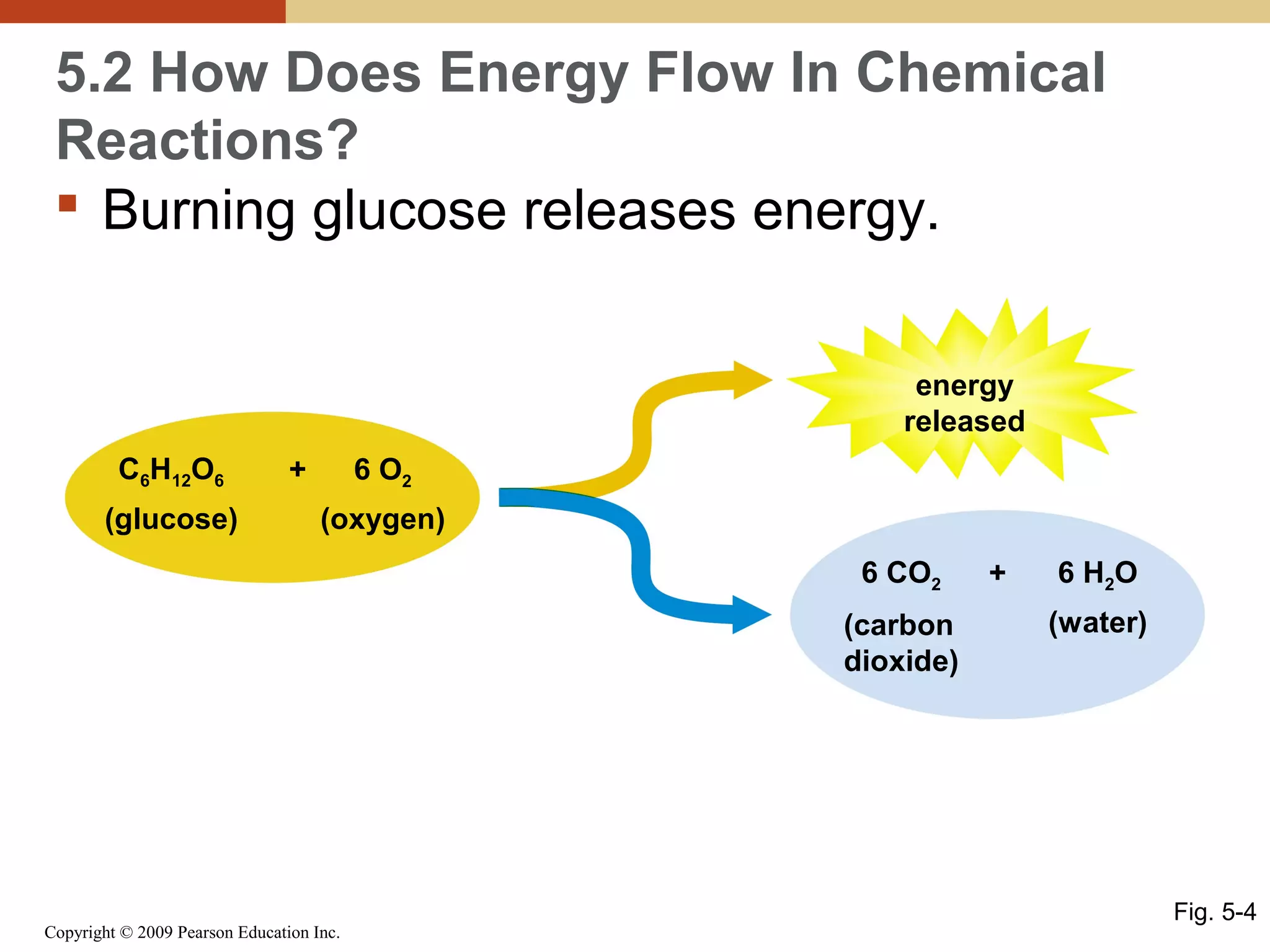
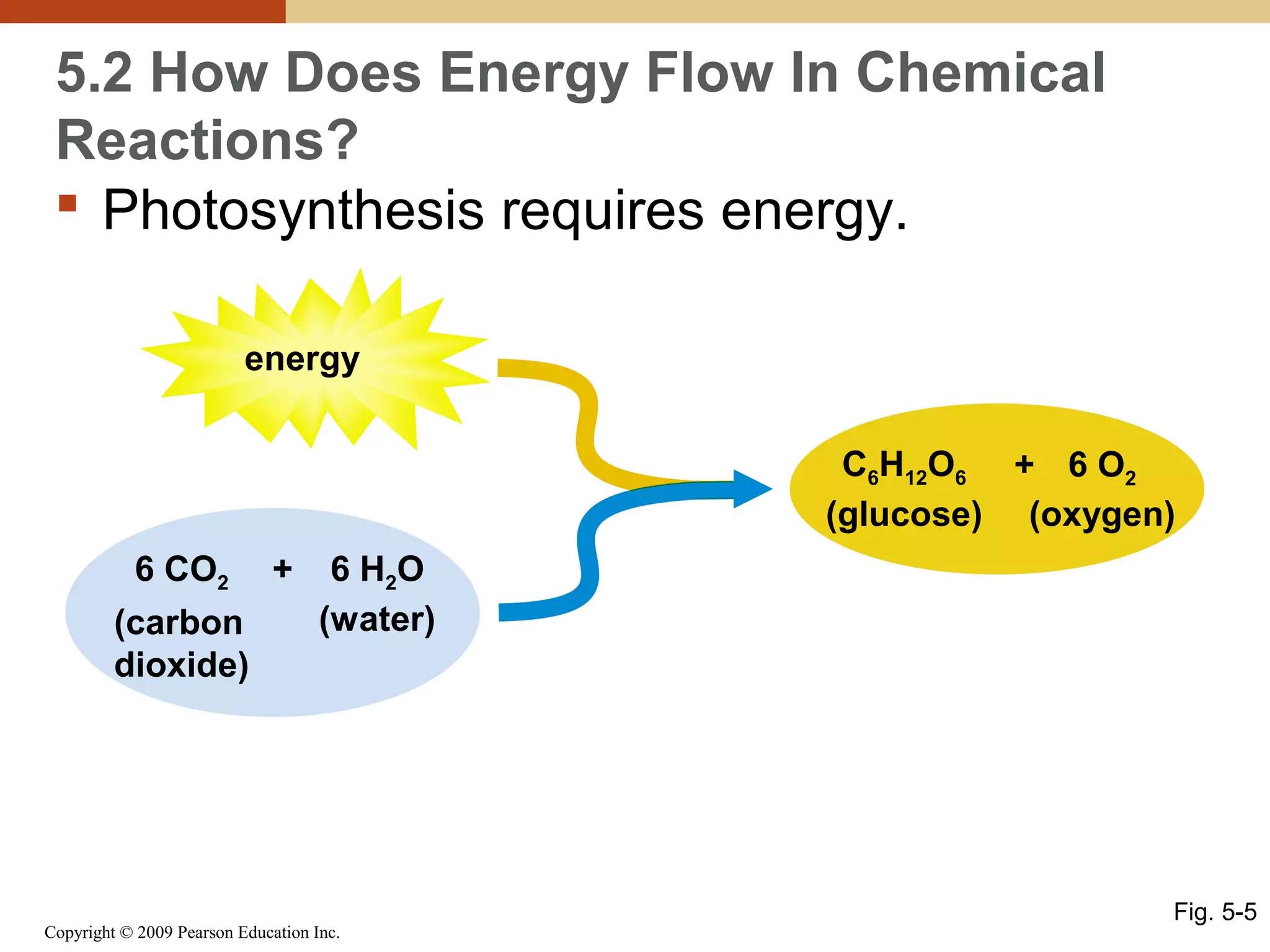
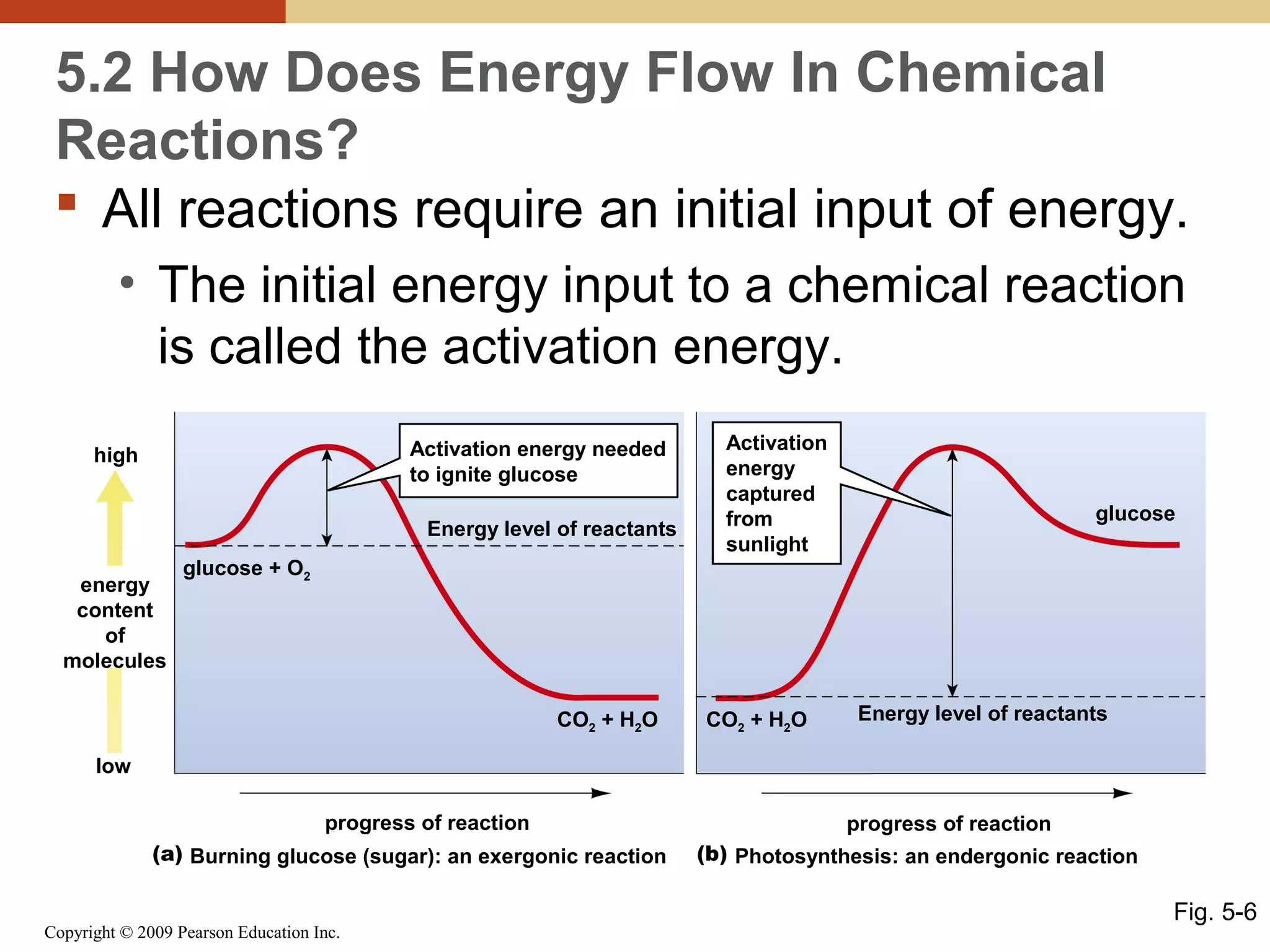
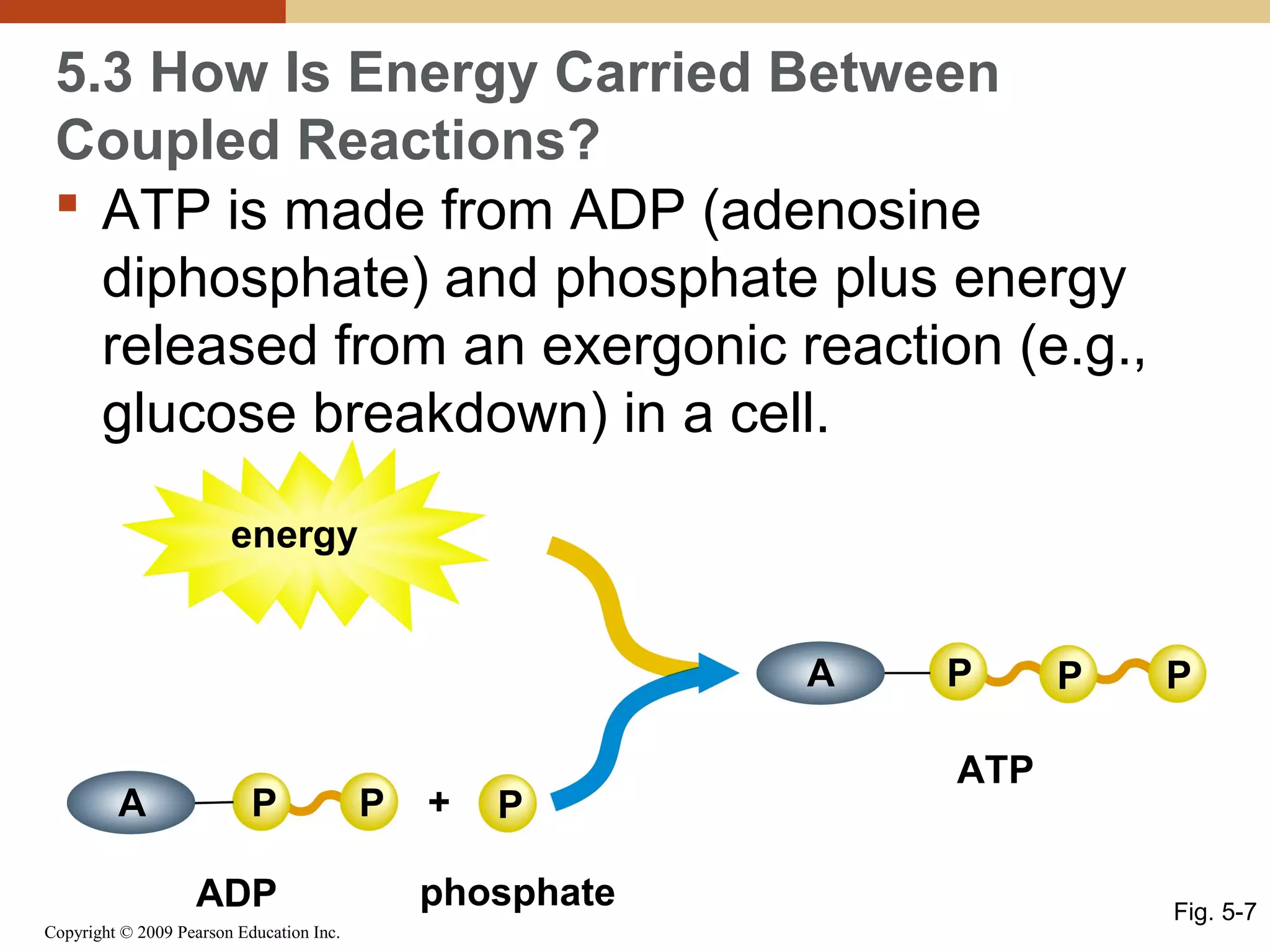
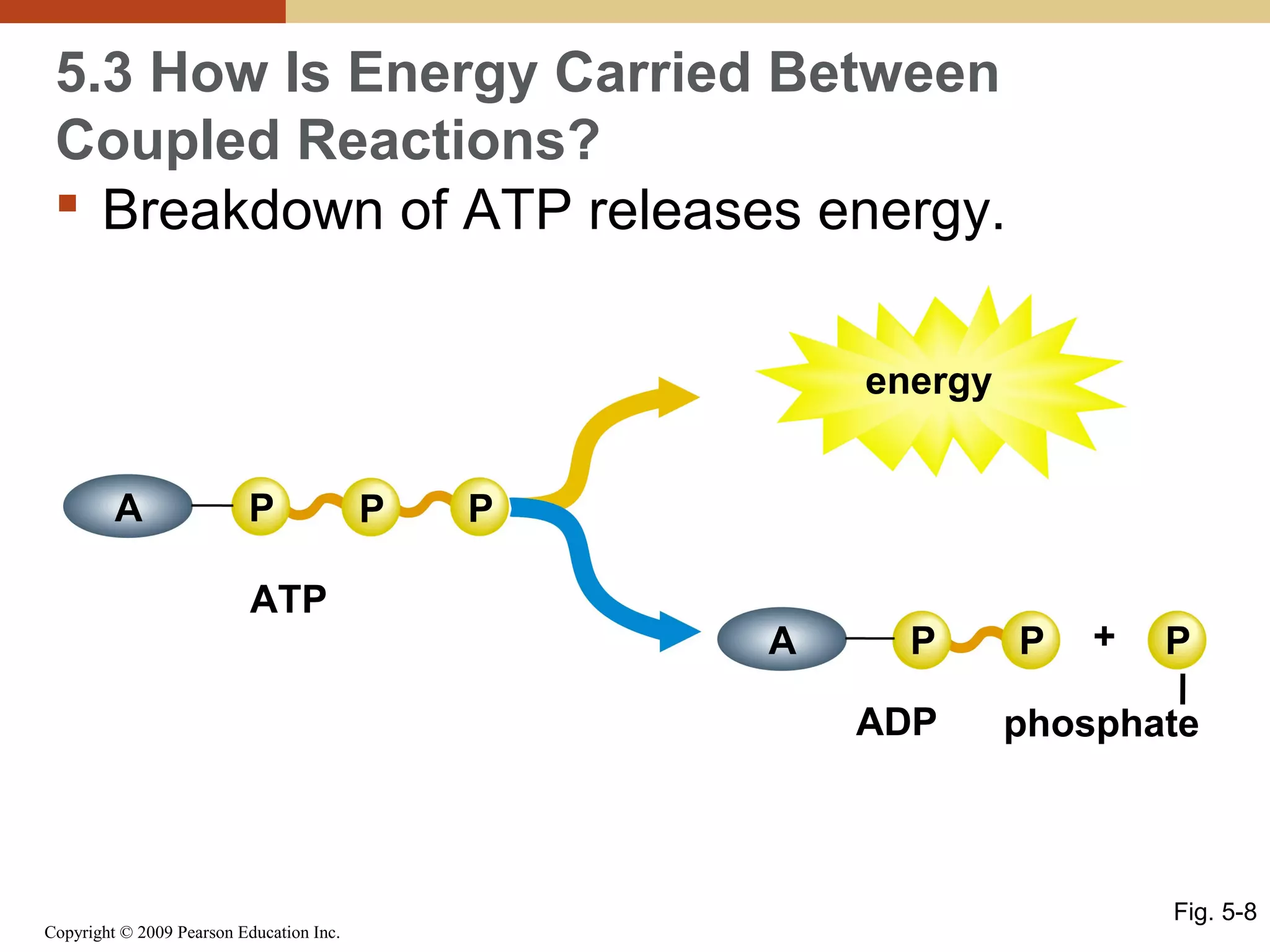
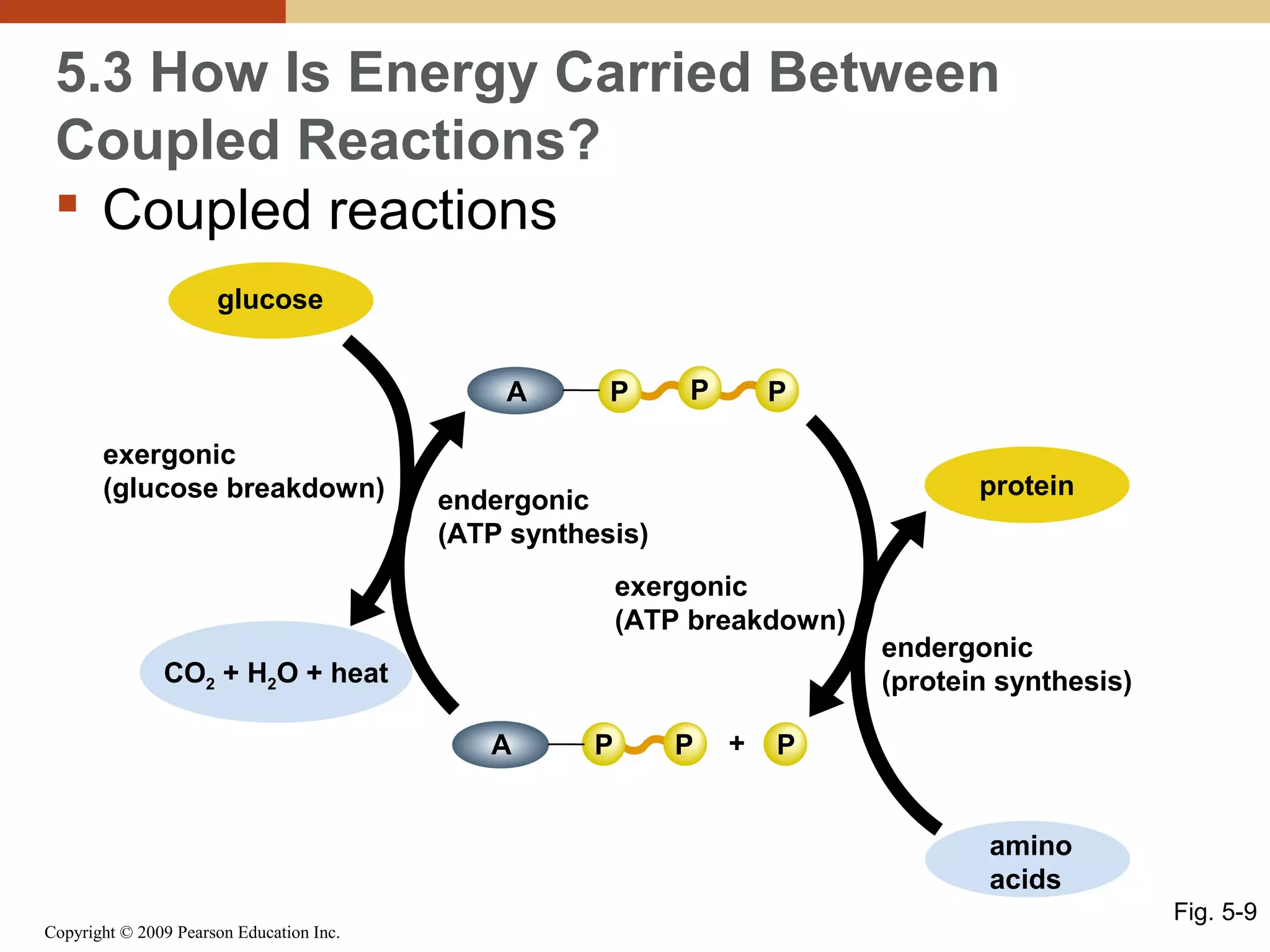
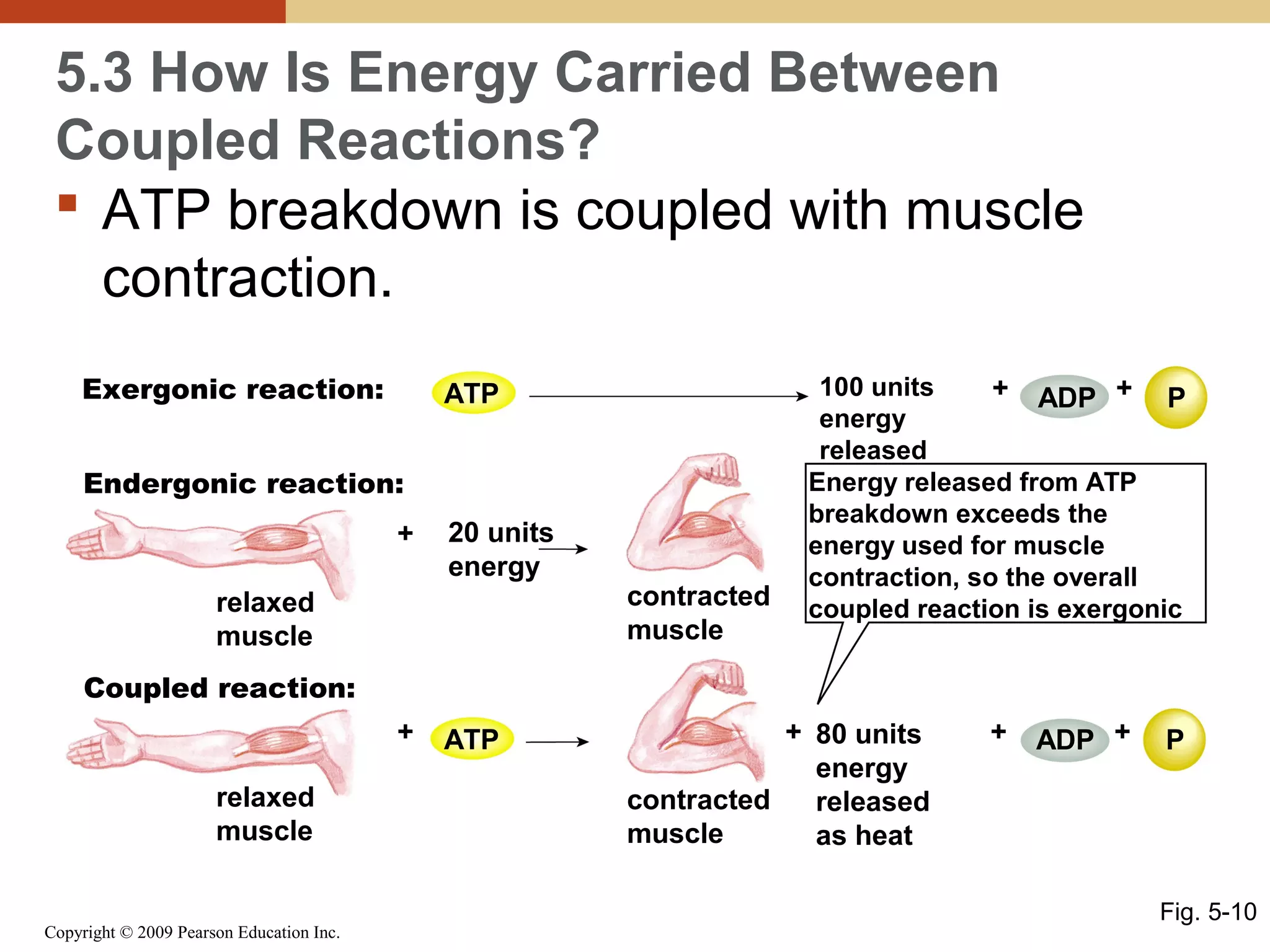
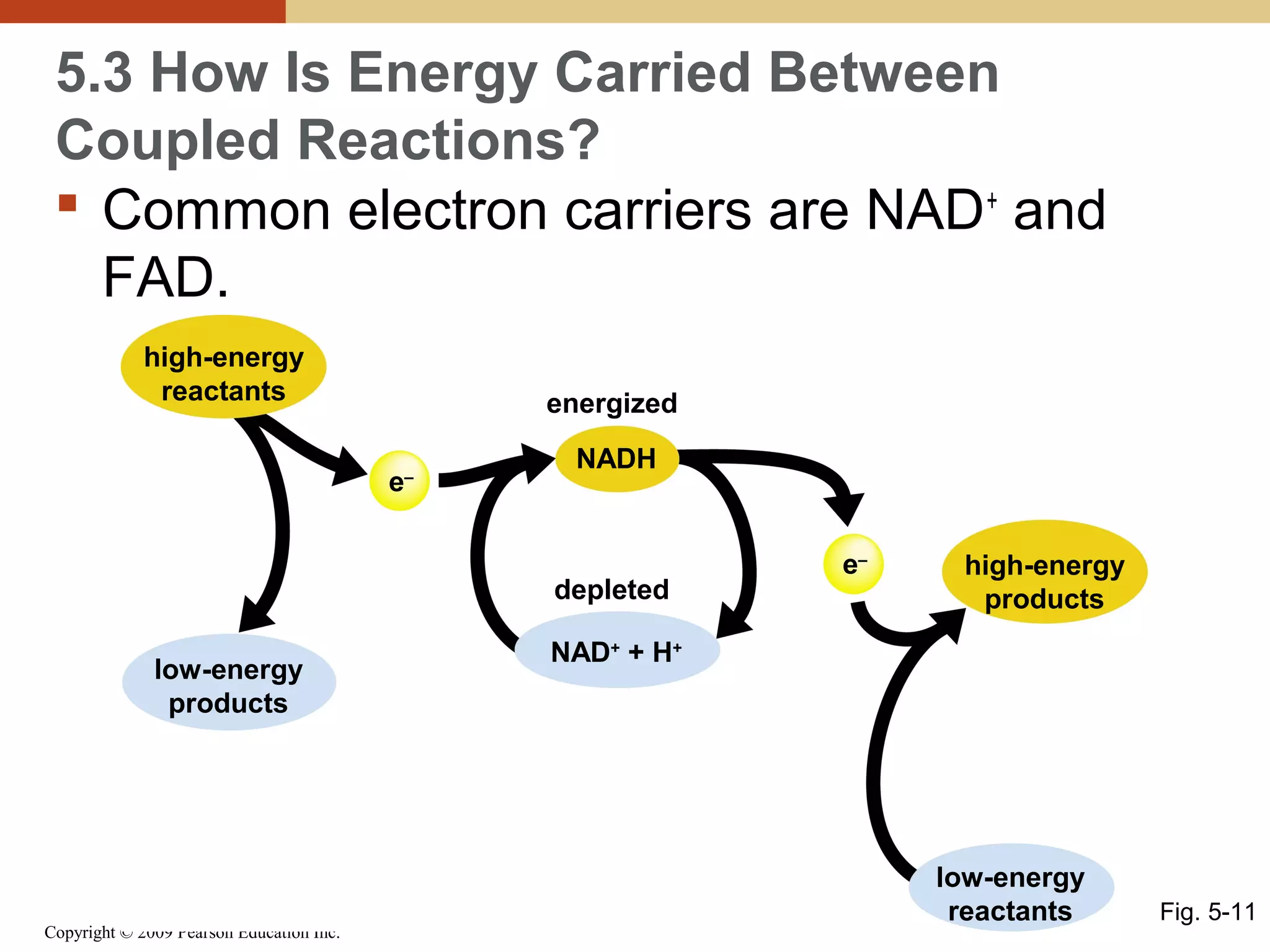
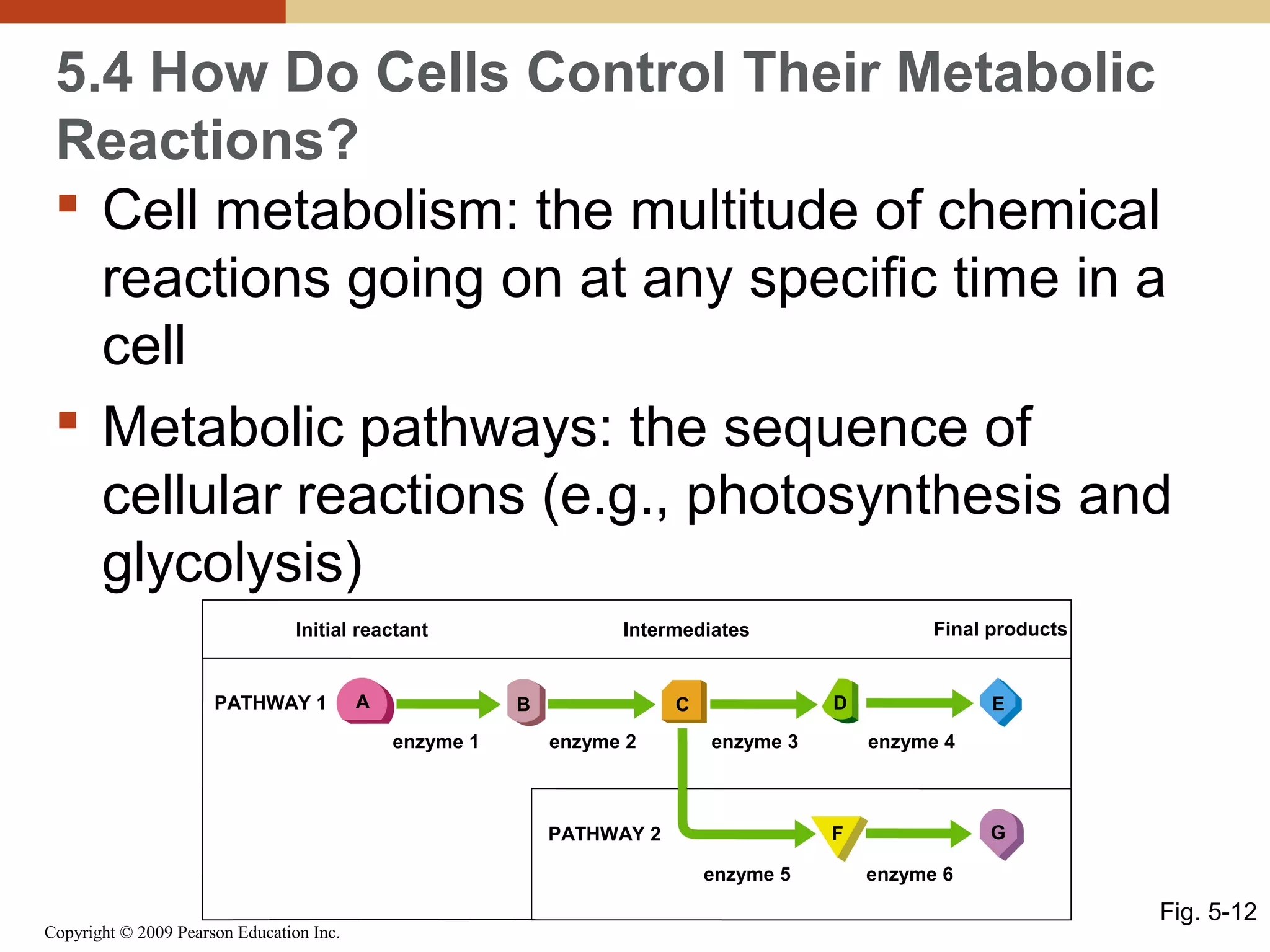
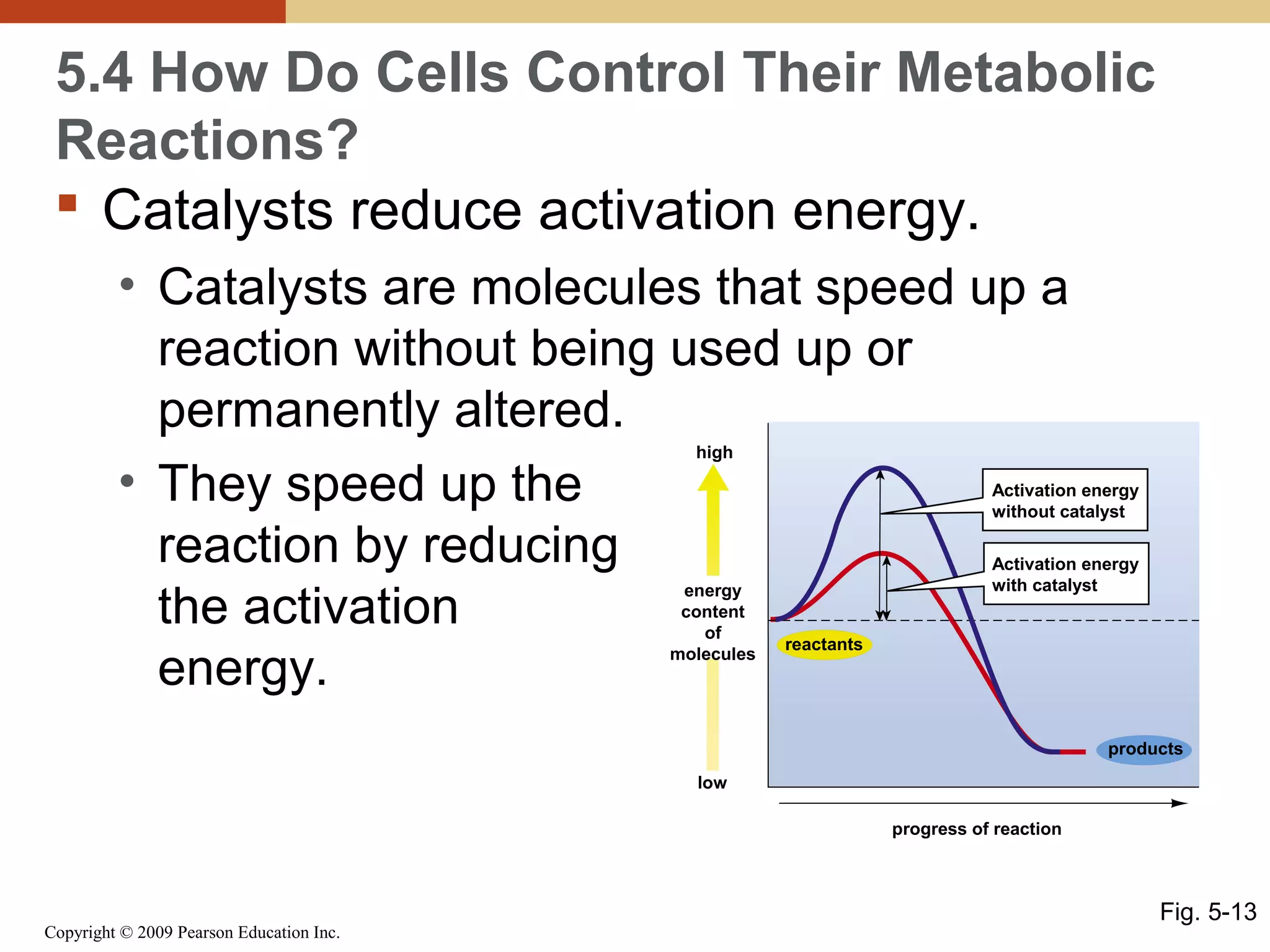
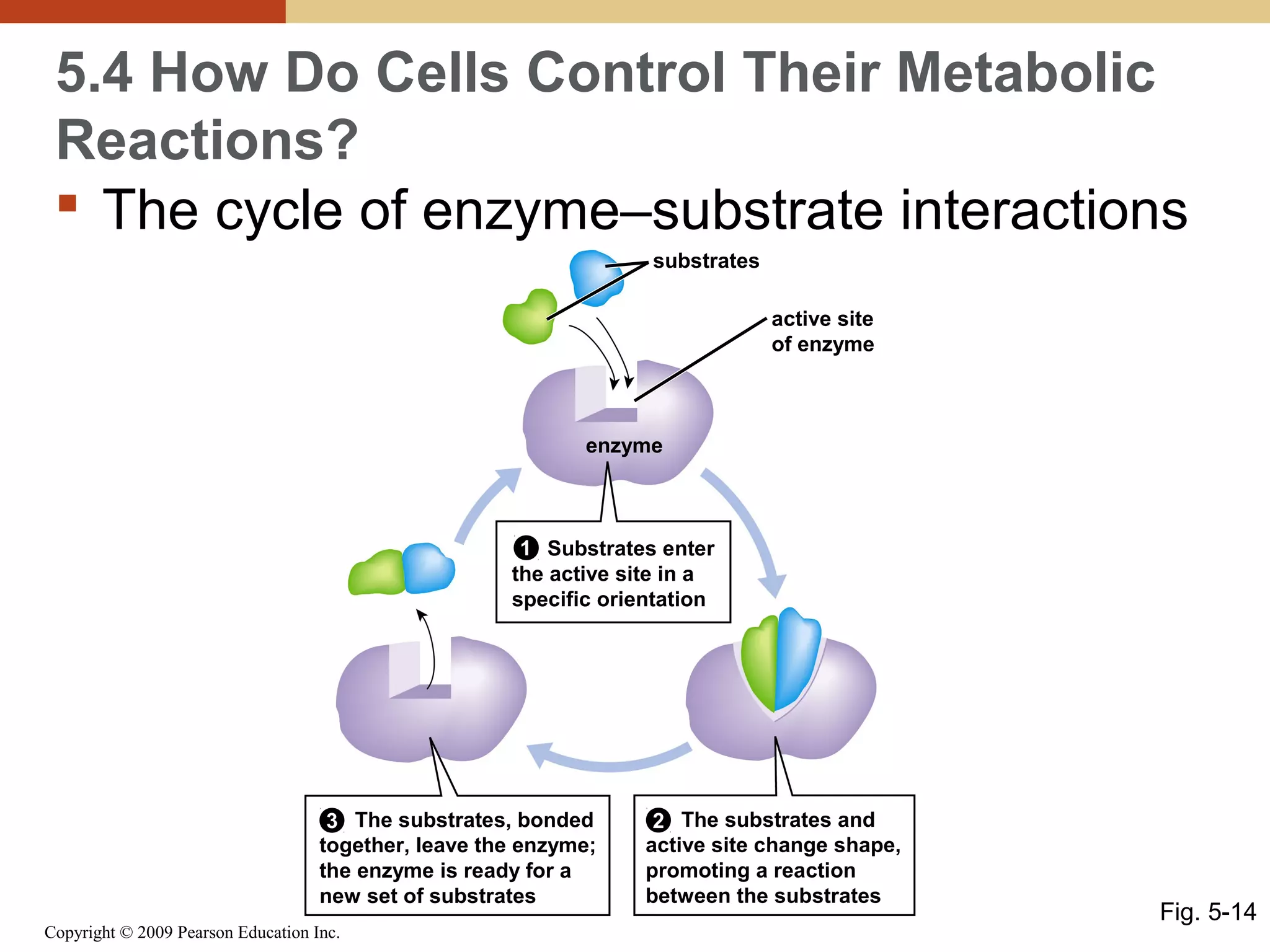
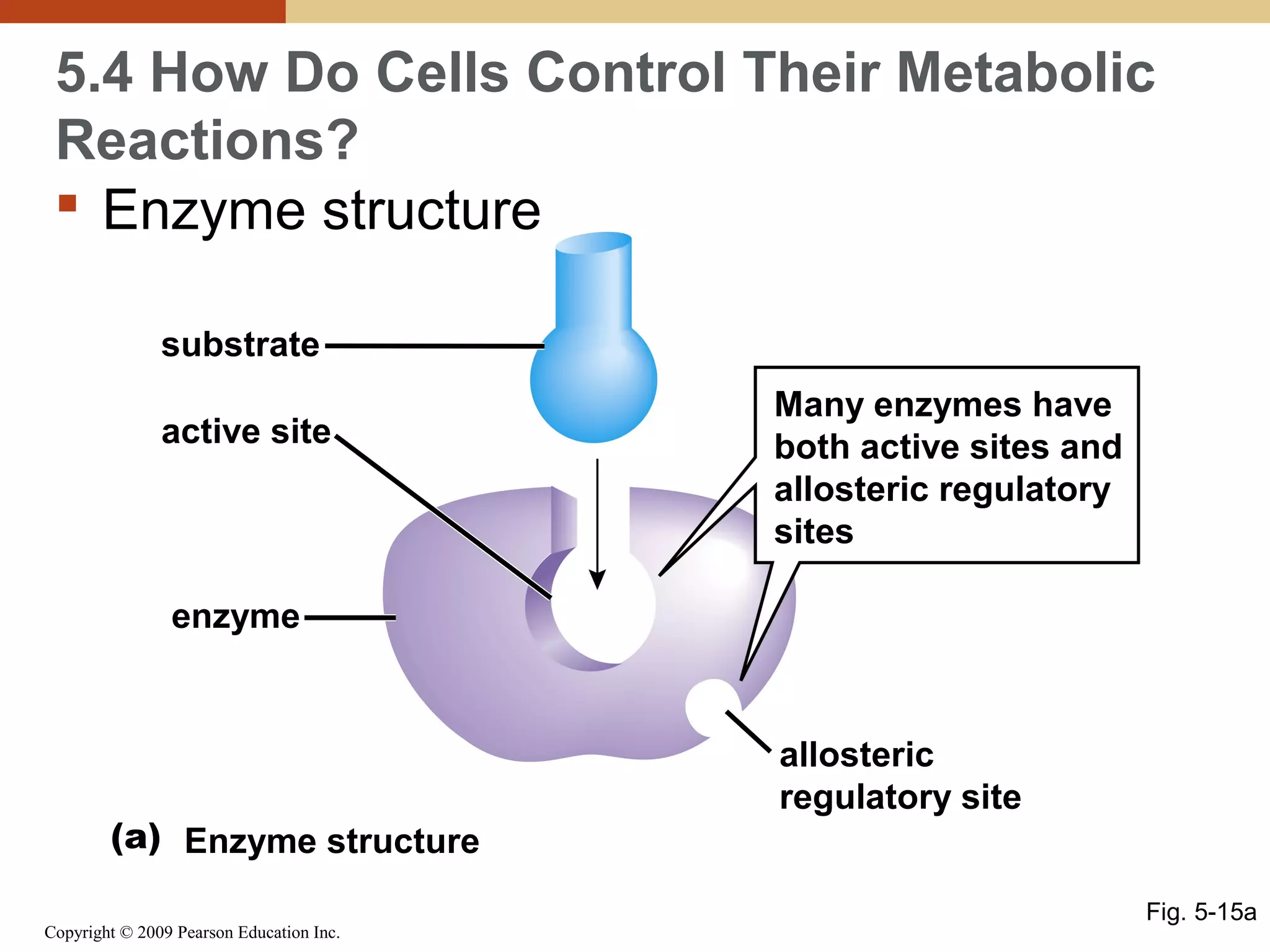
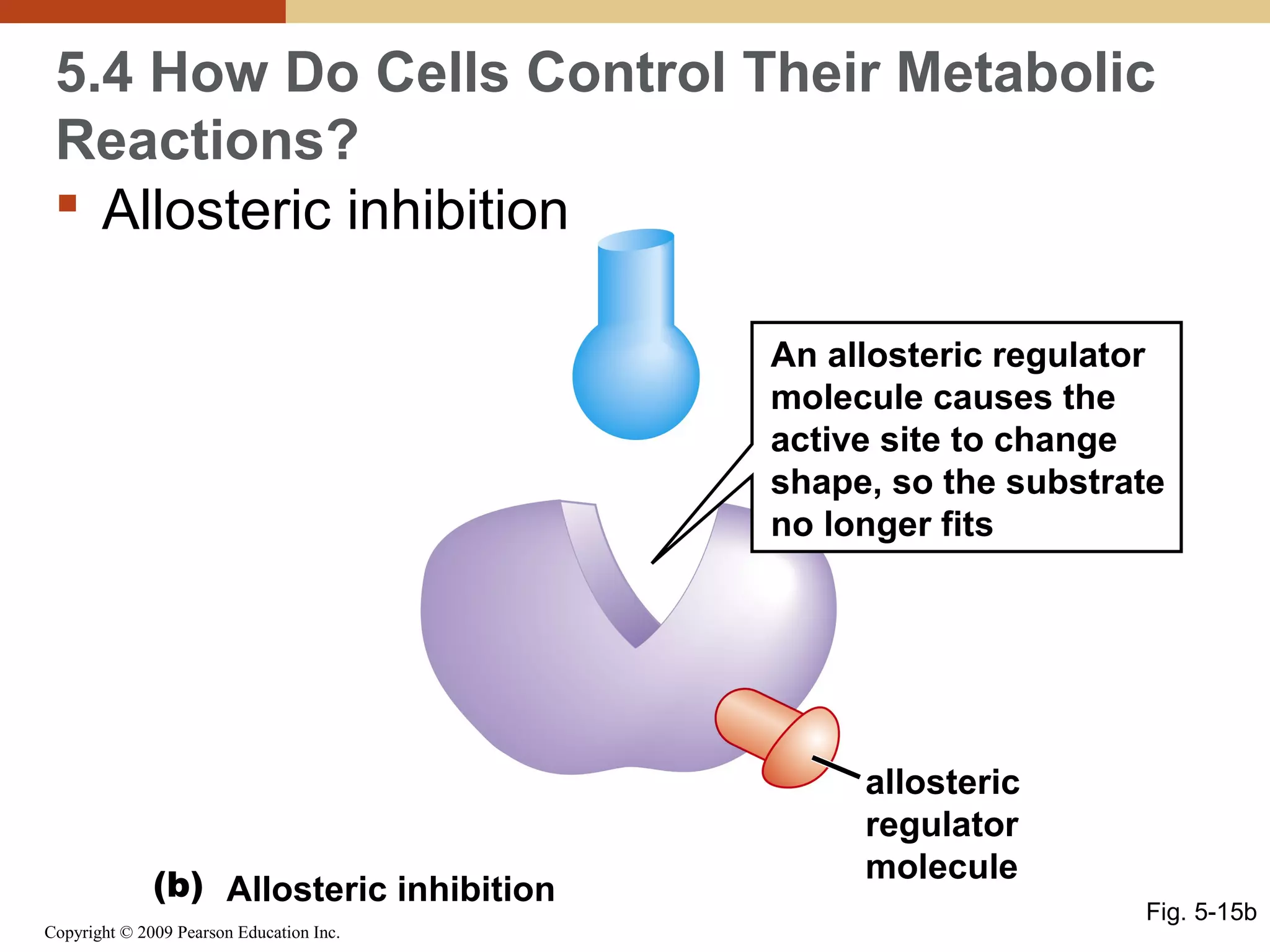
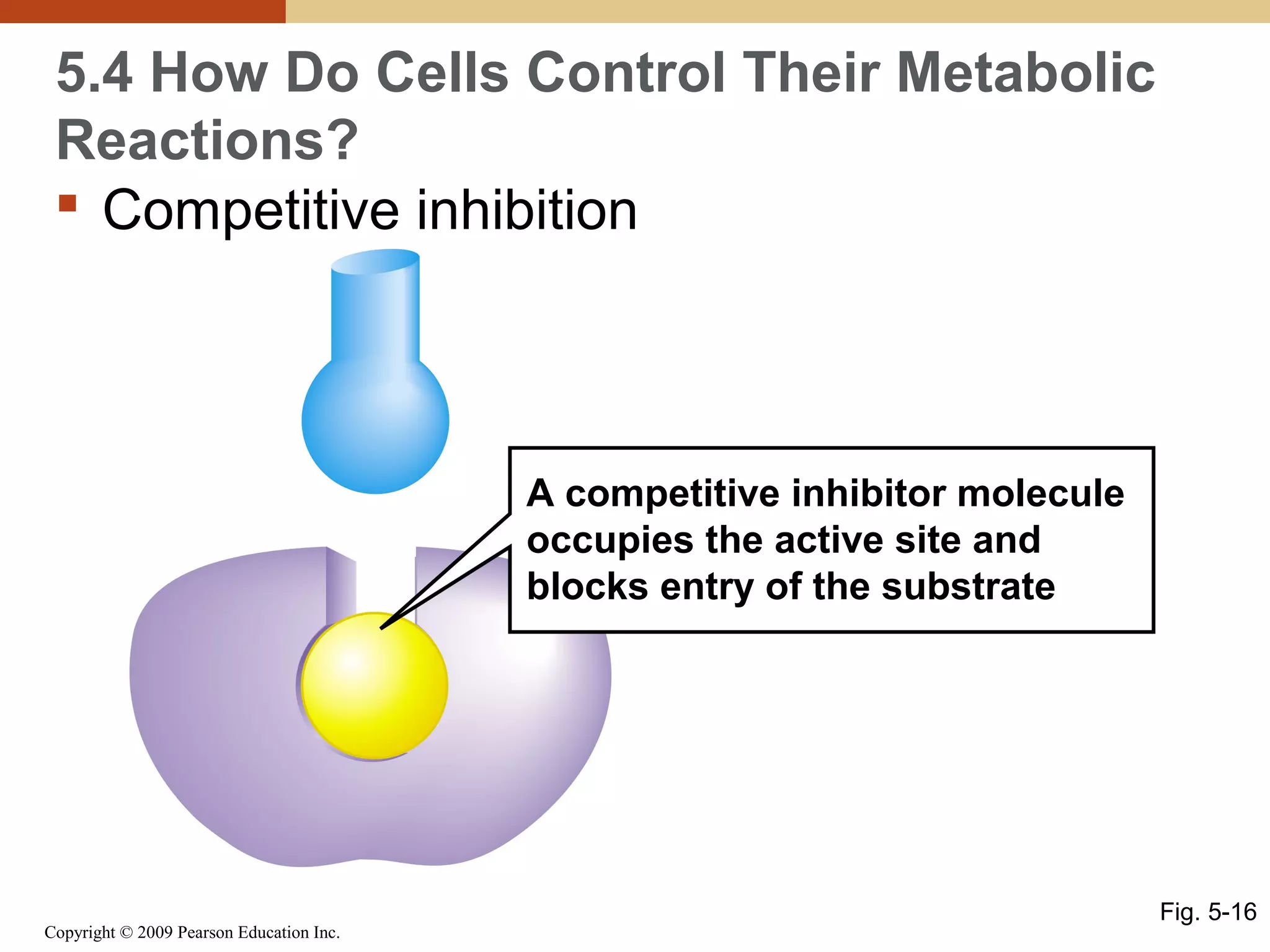
This document discusses energy flow in cells. It explains that energy is required to drive chemical reactions in cells, and is provided by exergonic reactions and carried by molecules like ATP and NADH to power endergonic reactions. Enzymes control metabolic reactions by reducing their activation energy. Cells regulate metabolism by controlling enzyme activity through allosteric regulation and competitive inhibition, allowing enzymes to be turned on and off.