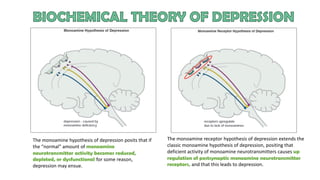
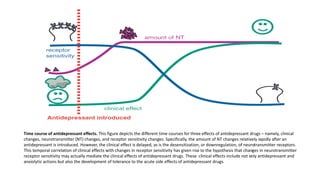
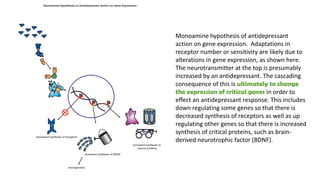
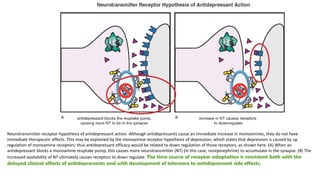
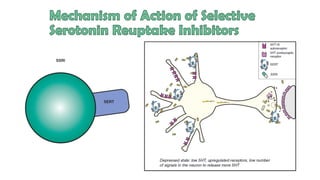
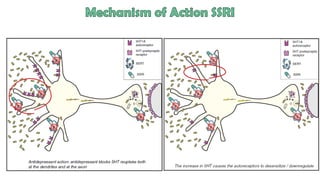
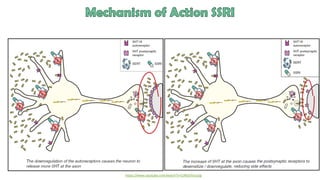
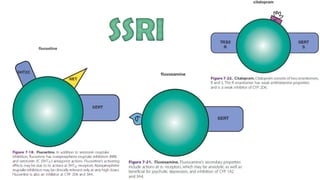
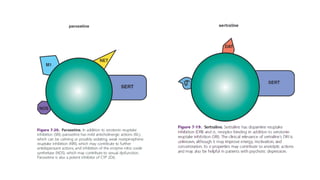
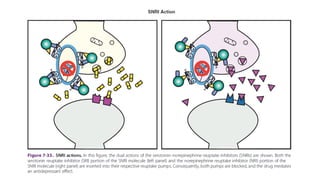
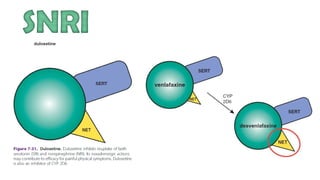
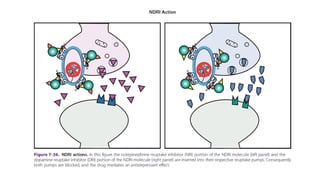
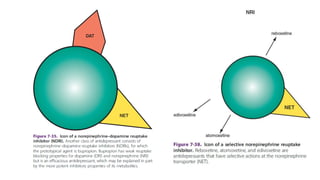
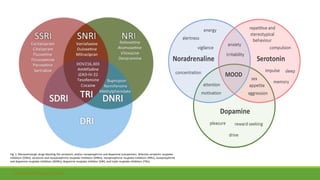
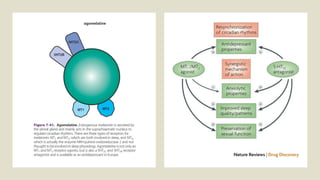
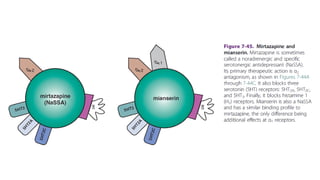
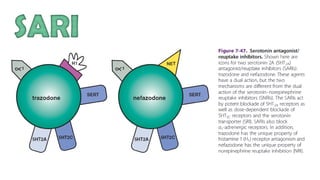
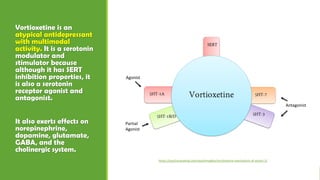
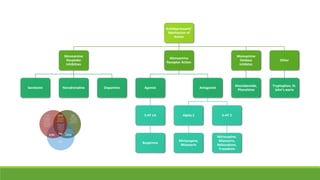
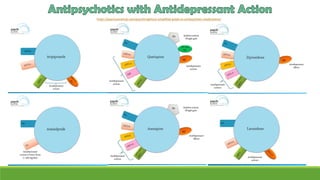
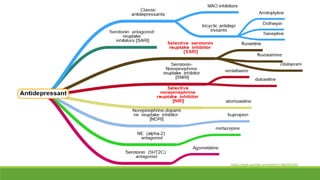
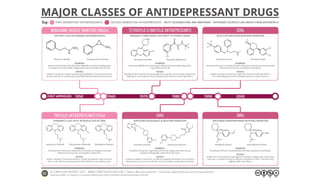
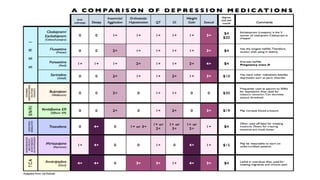
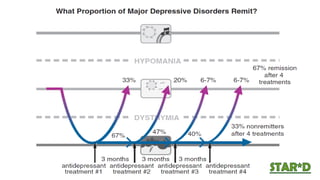
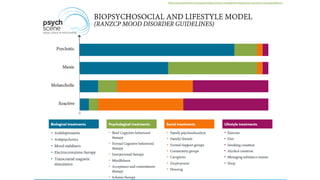
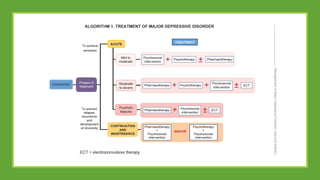
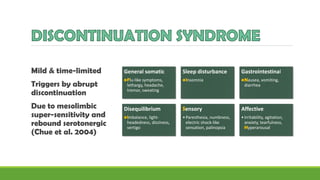
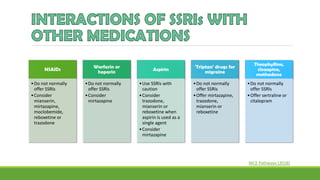
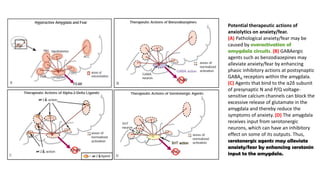
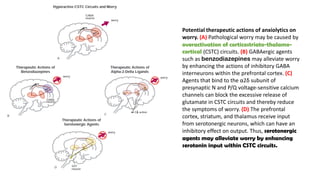
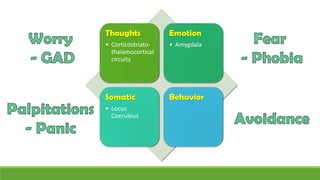
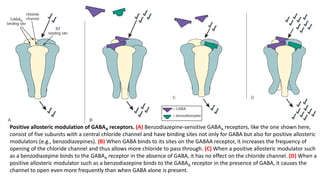
The document discusses several hypotheses regarding depression and the mechanisms of antidepressant medications. The monoamine hypothesis posits that reduced monoamine neurotransmitter activity can cause depression. The monoamine receptor hypothesis extends this by proposing that deficient monoamine activity leads to upregulation of postsynaptic receptors. Antidepressants are thought to work by increasing monoamine levels initially, but their therapeutic effects are delayed and correlated with later downregulation of neurotransmitter receptors. Changes in gene expression may underlie these receptor adaptations.