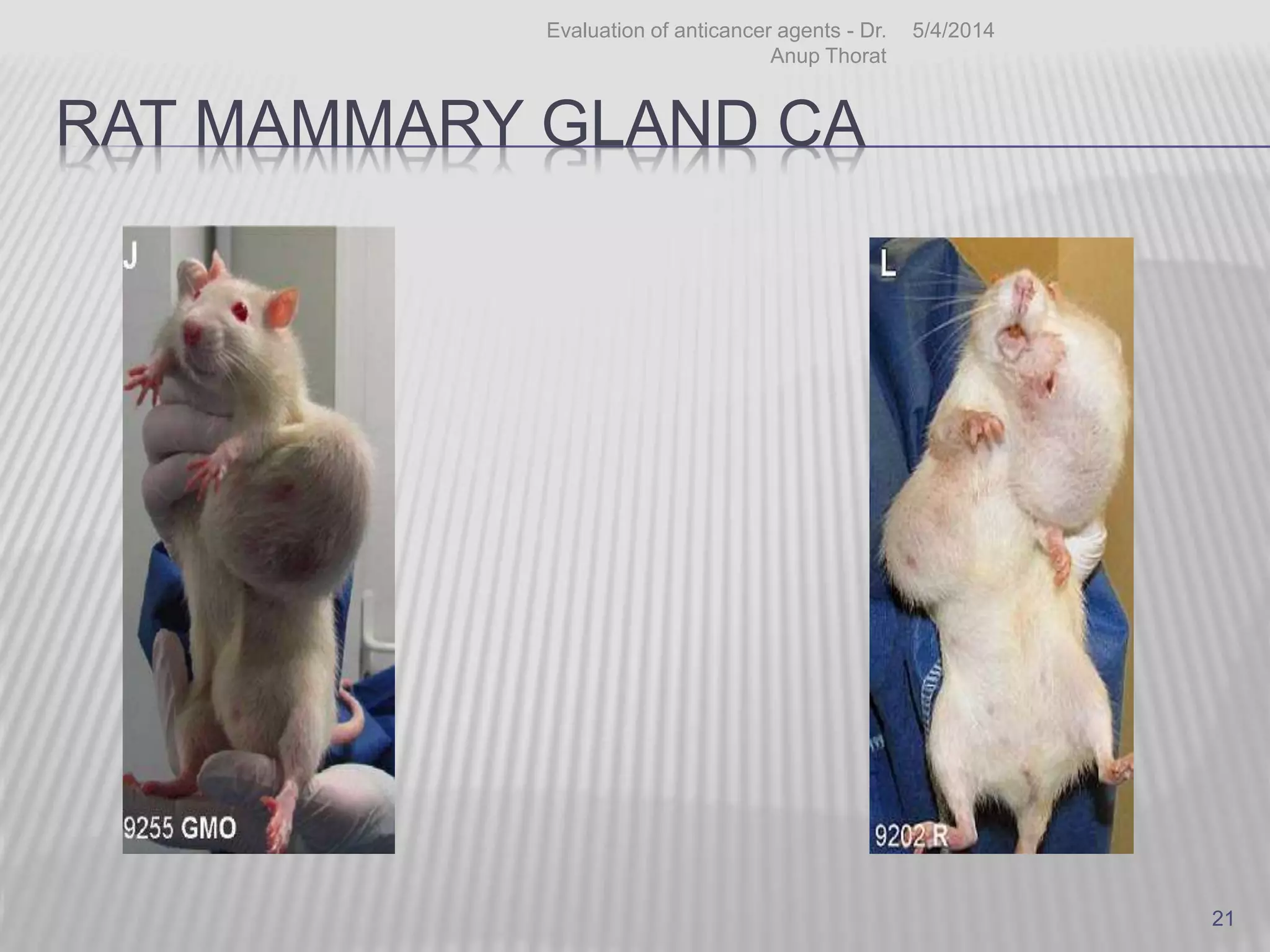
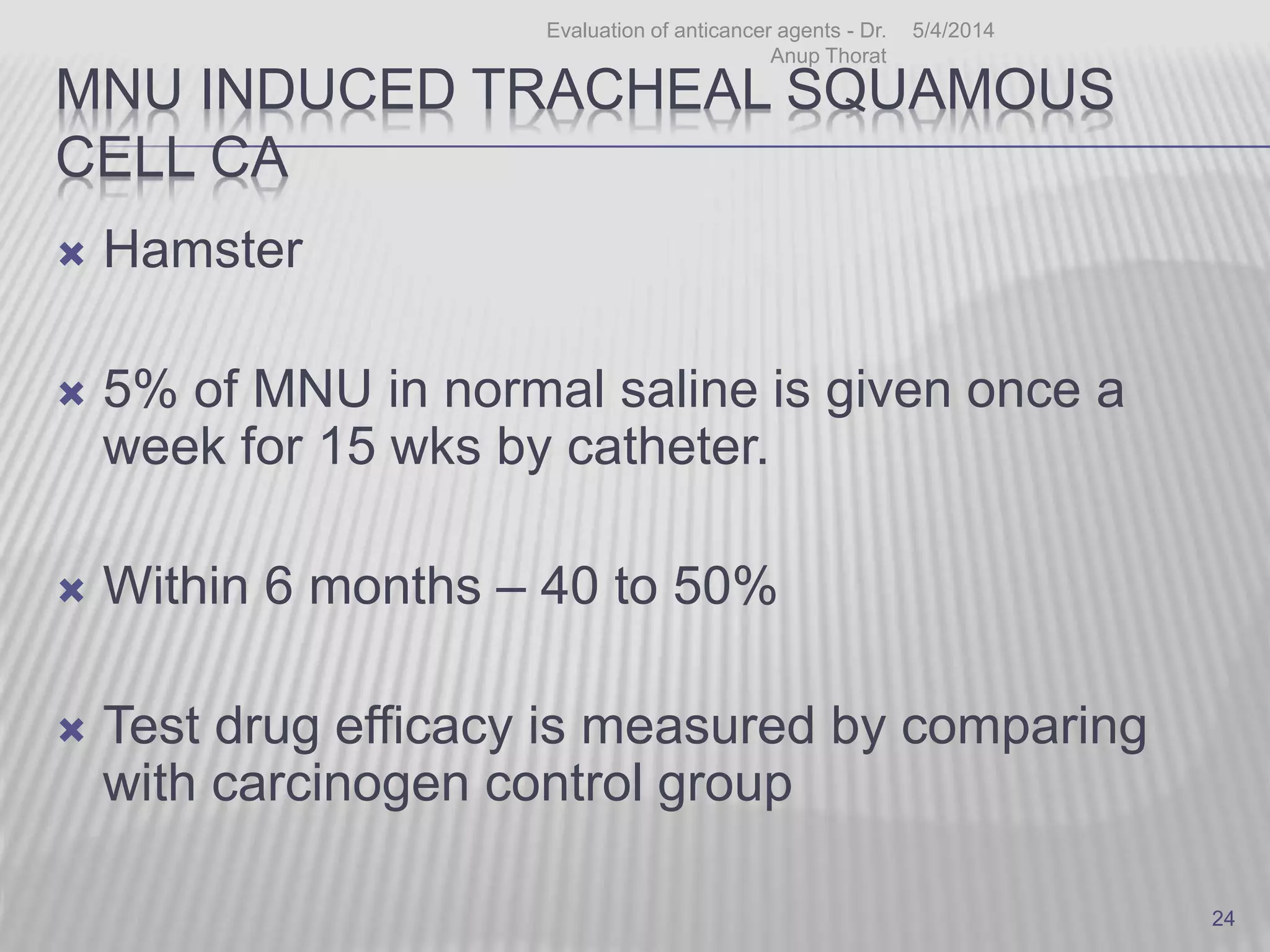
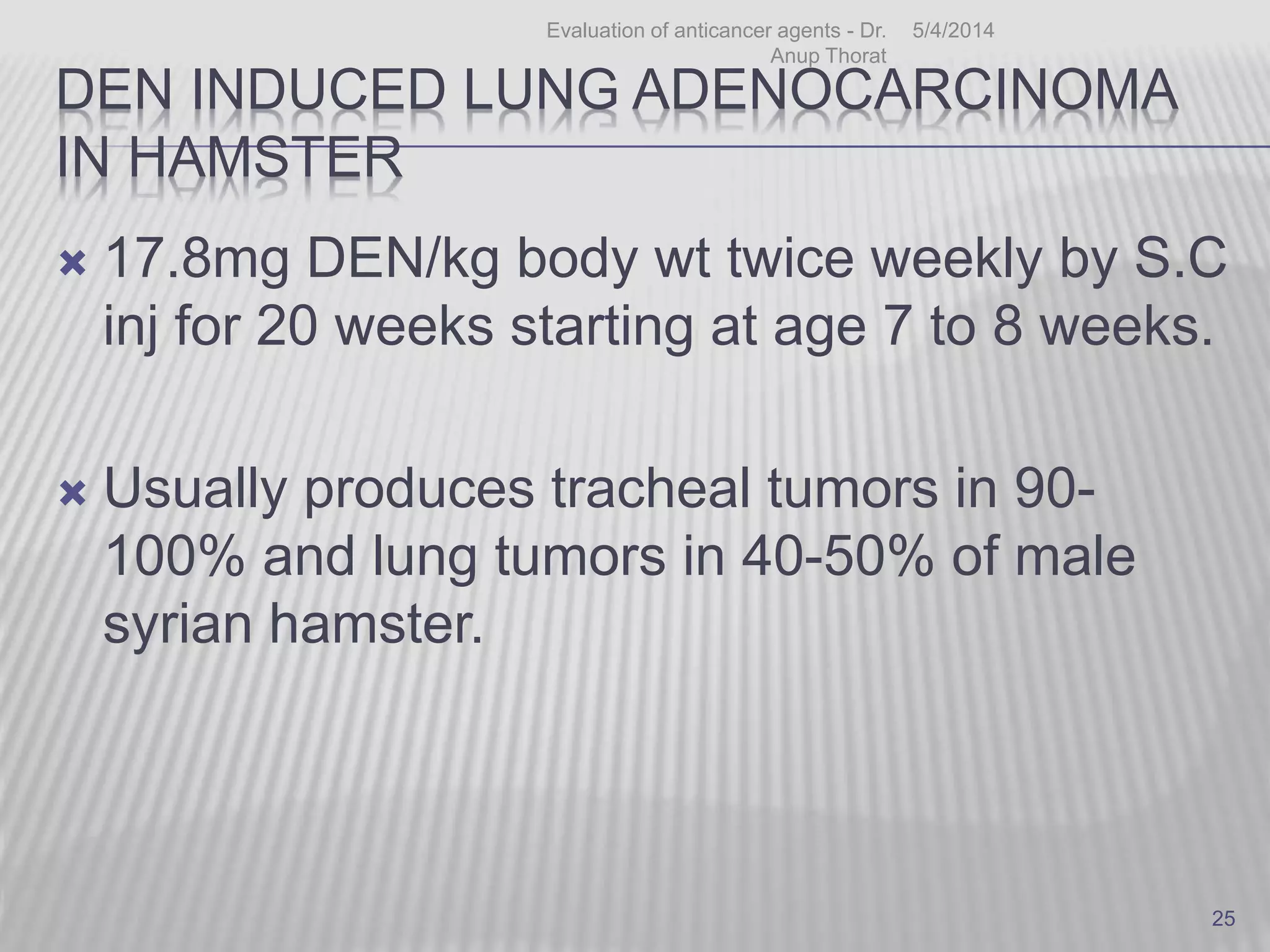
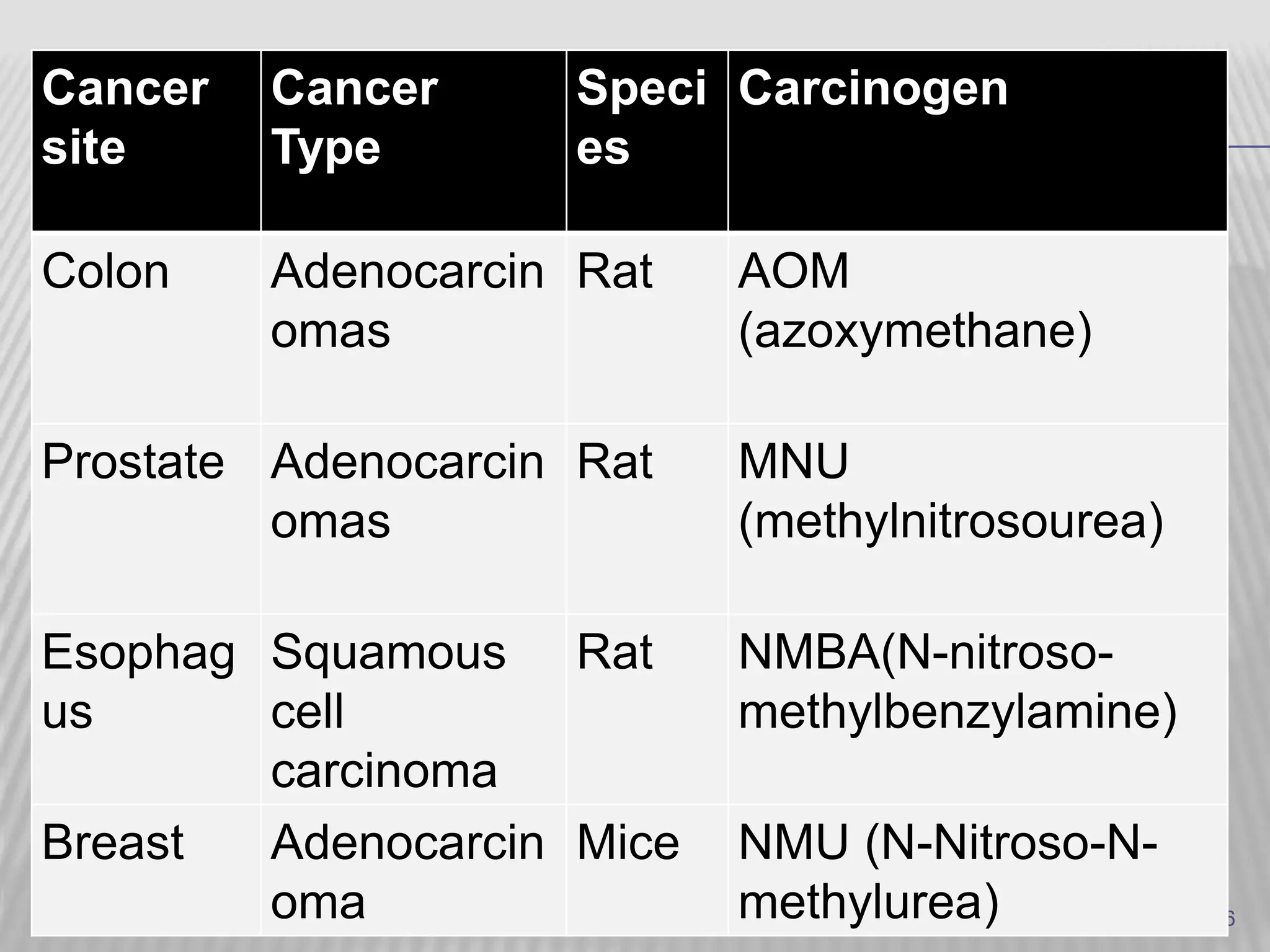
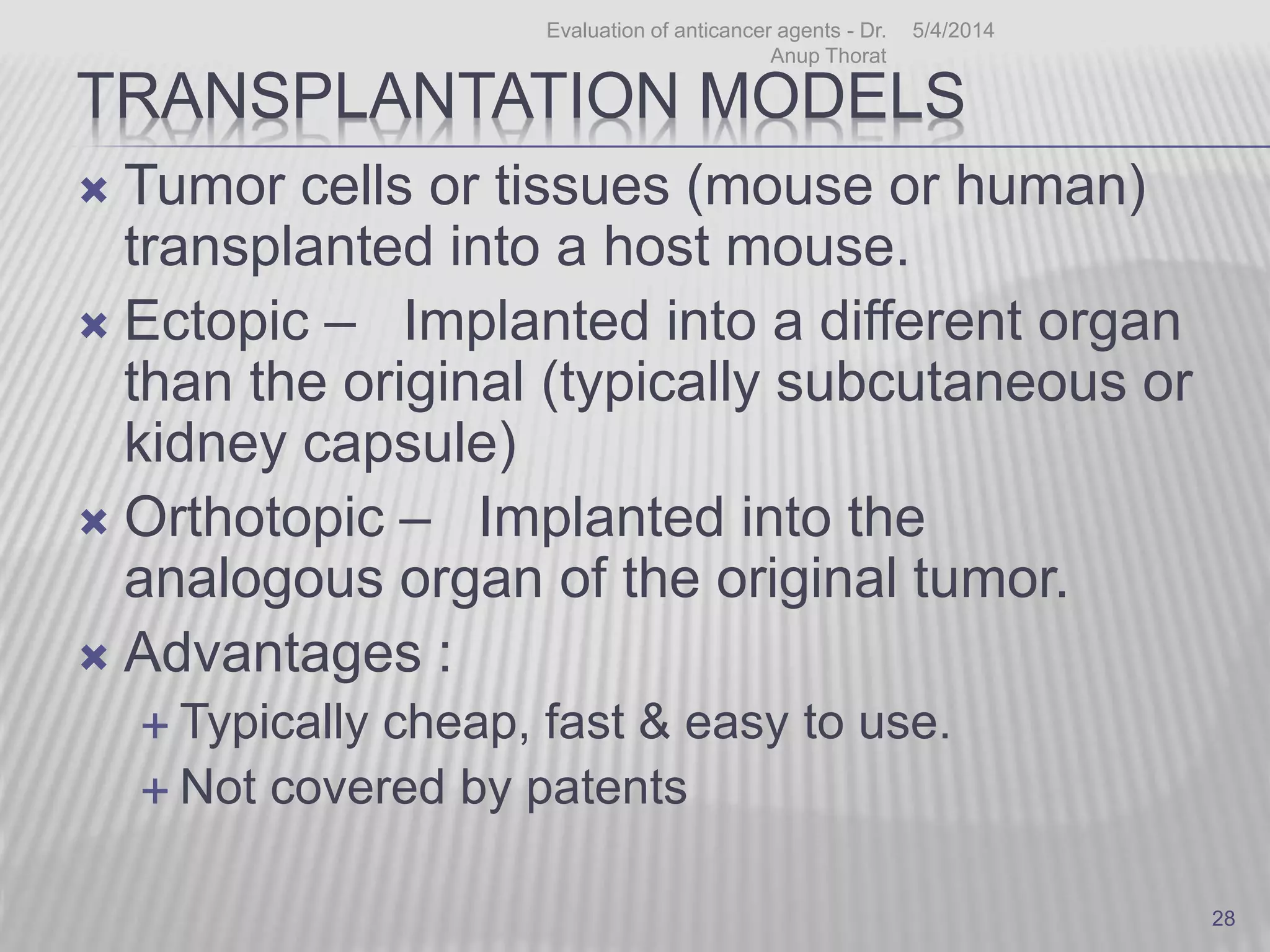
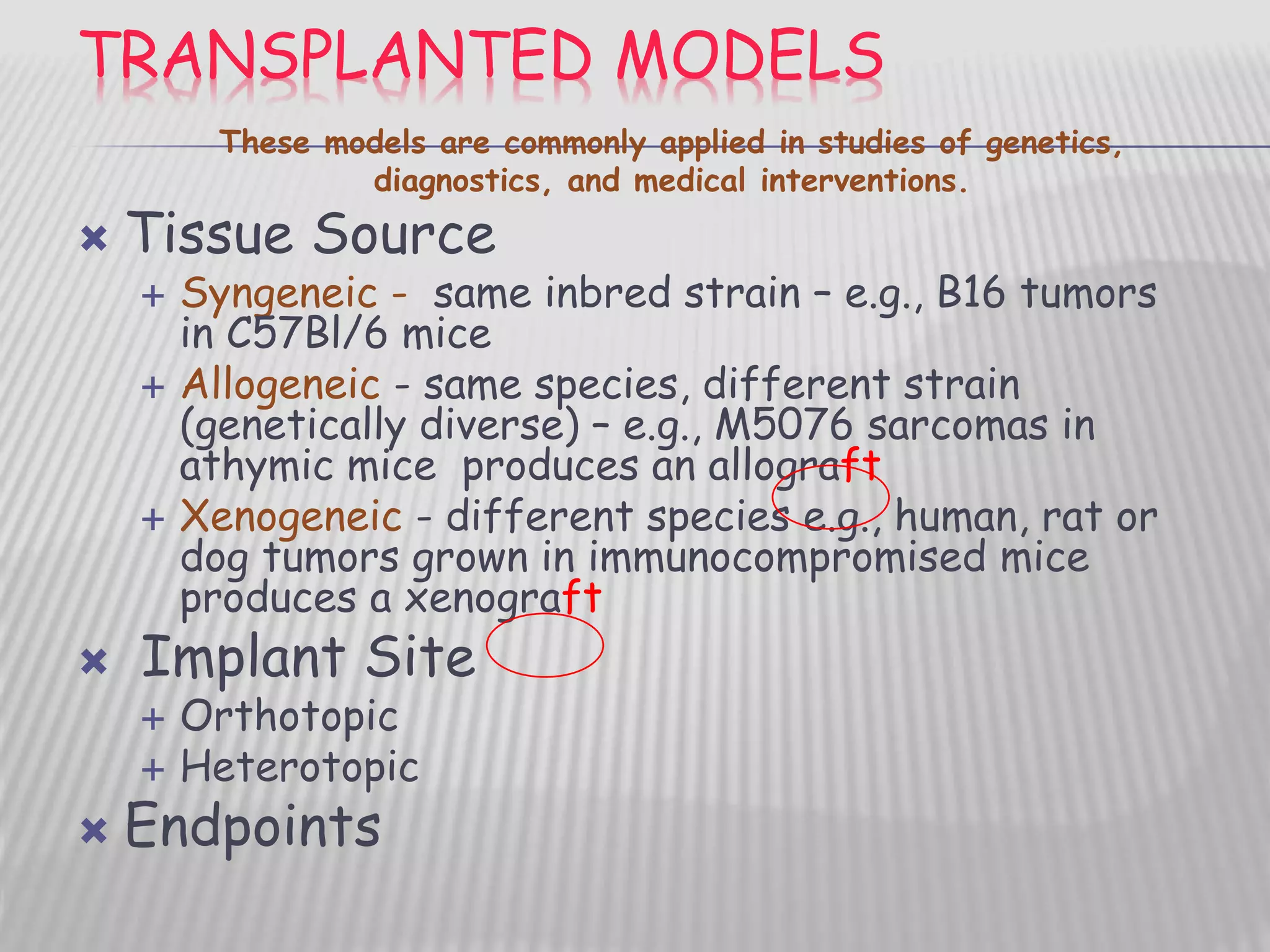
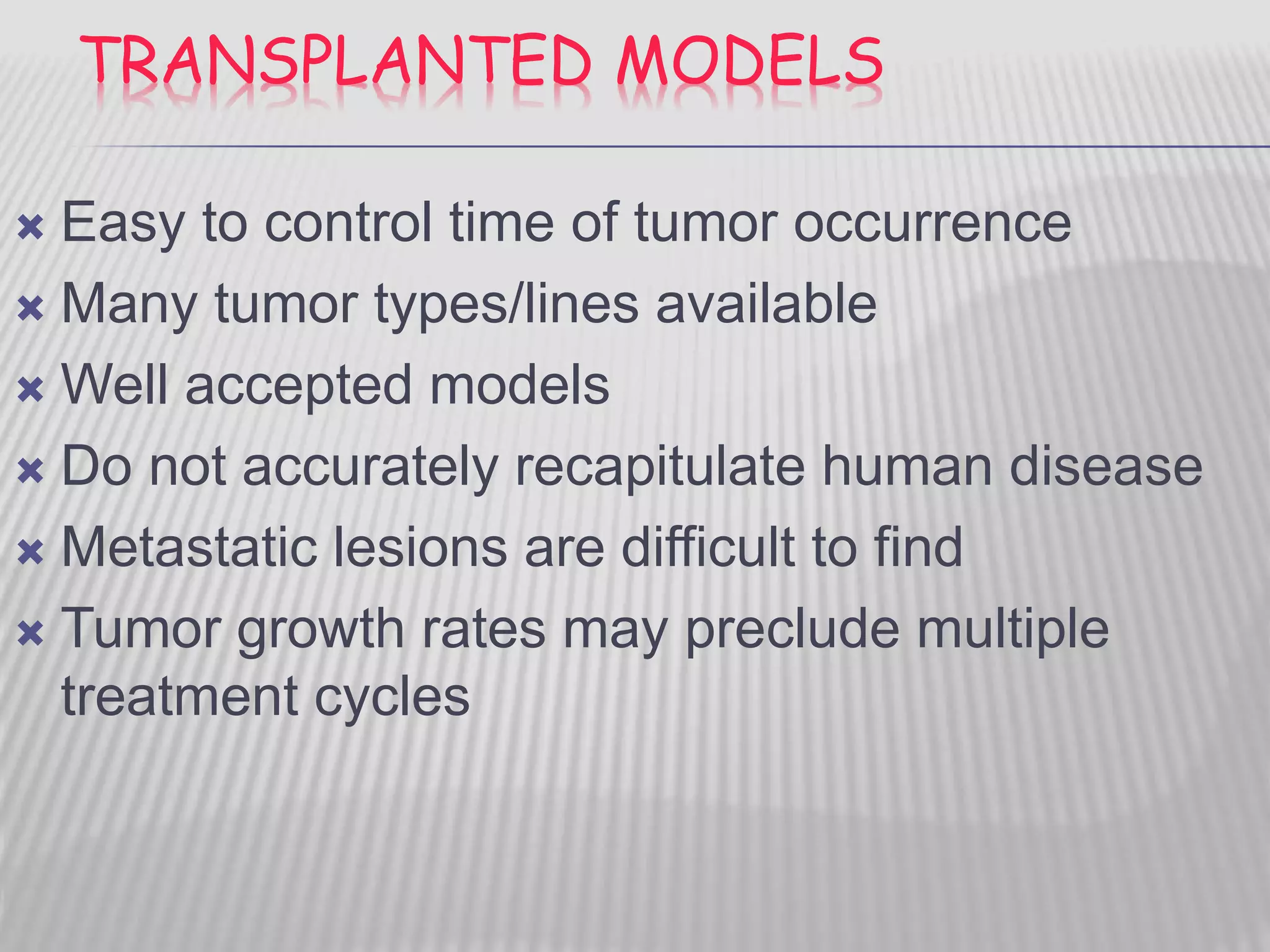
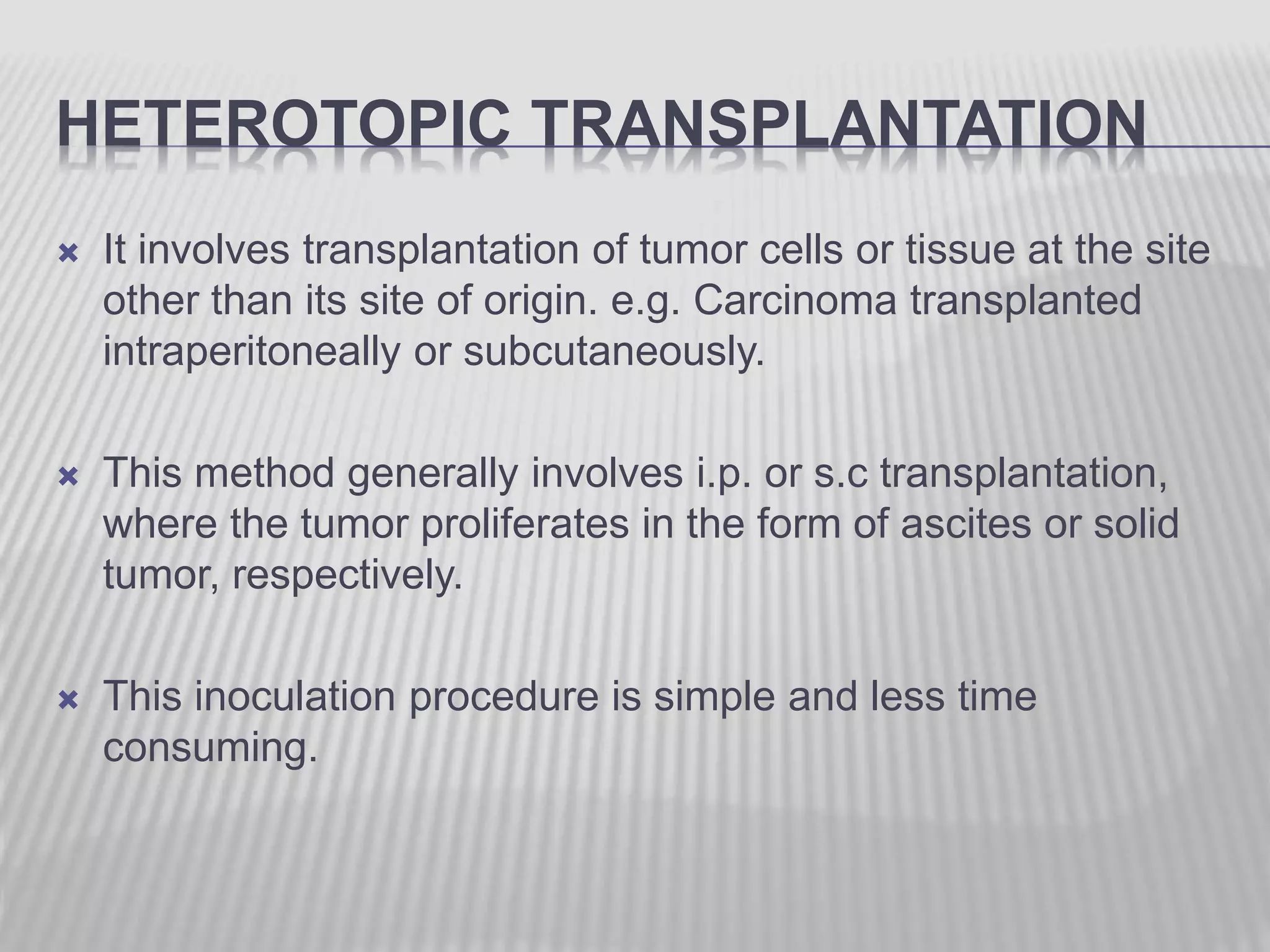
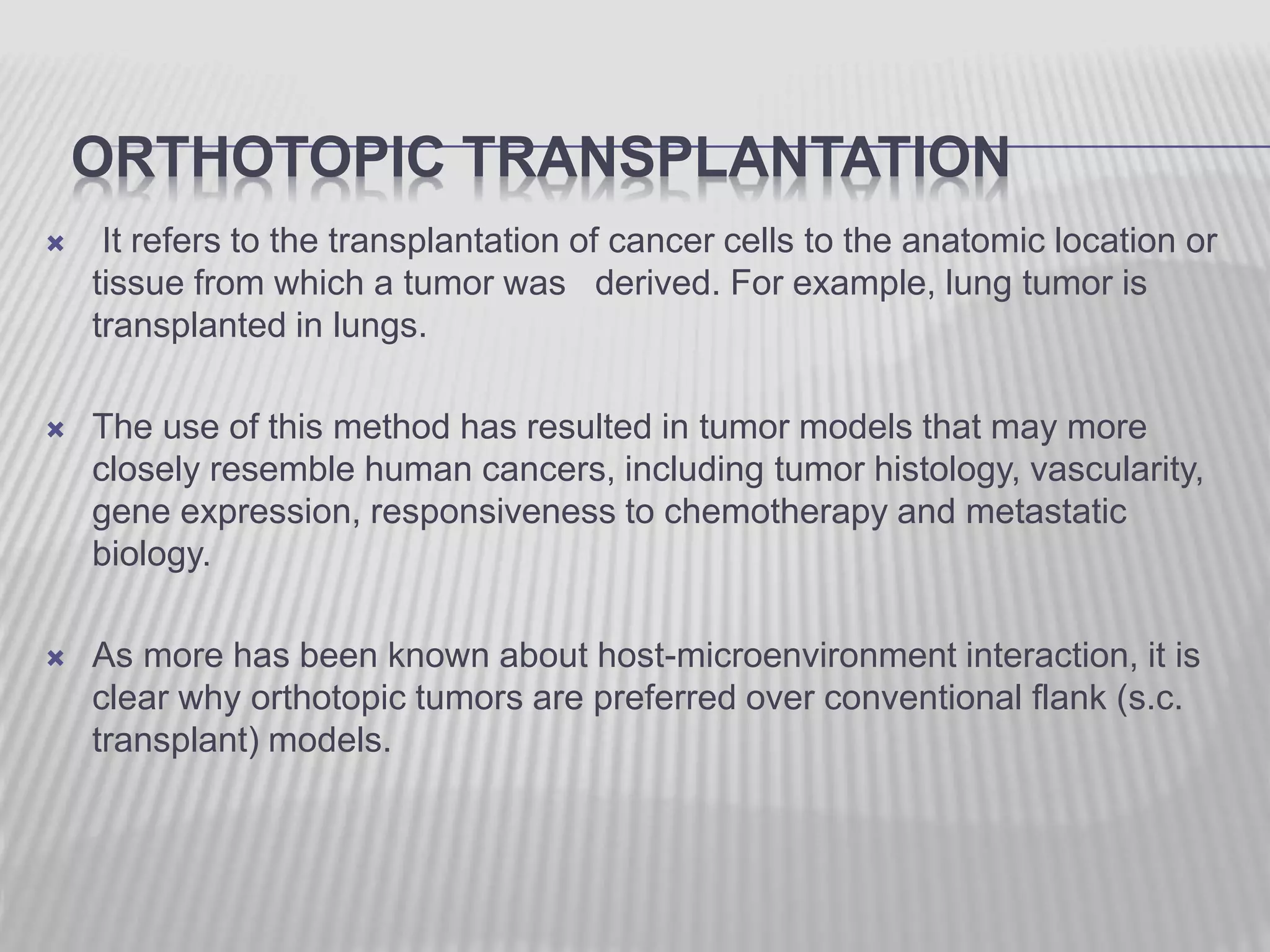
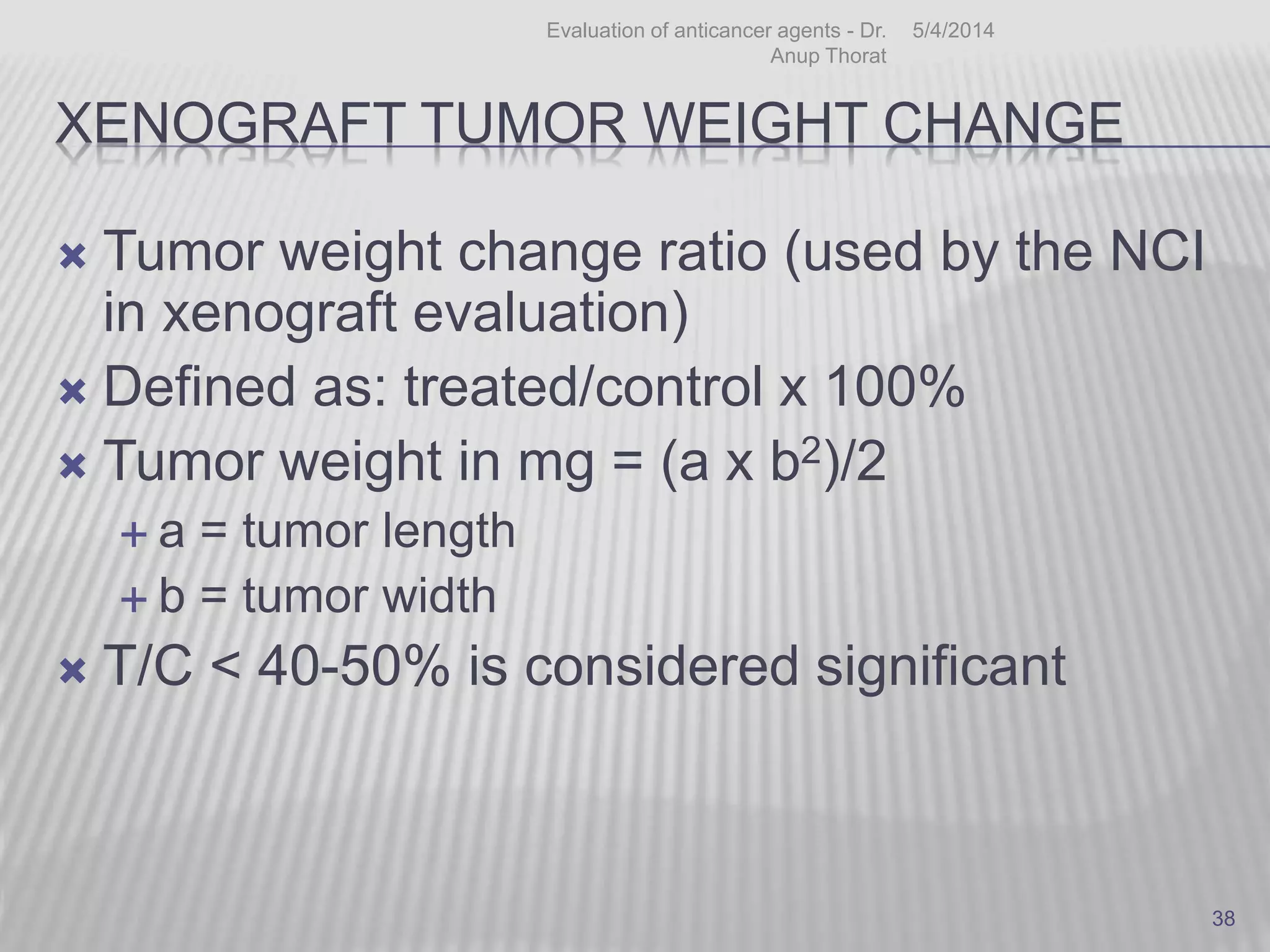
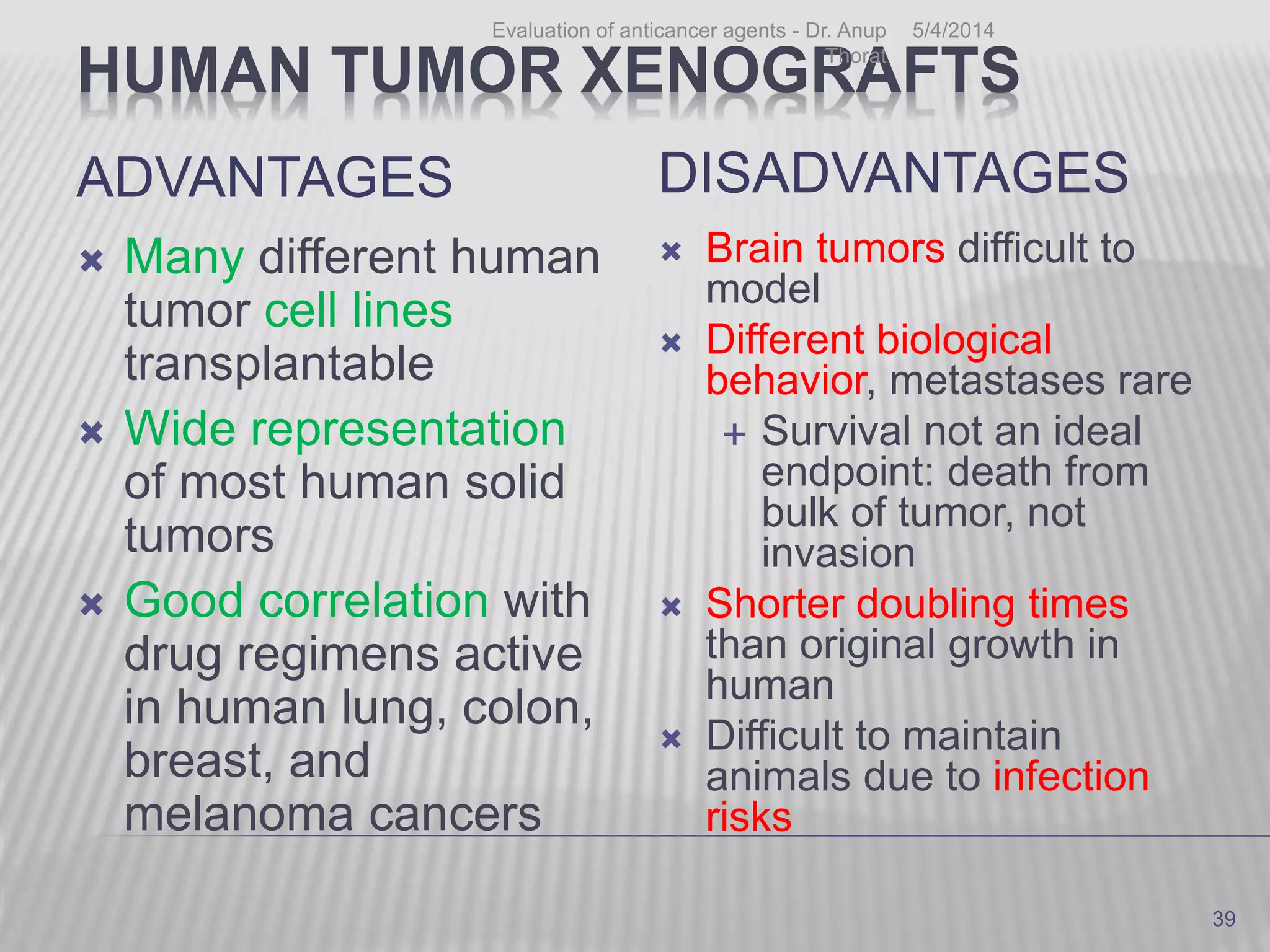
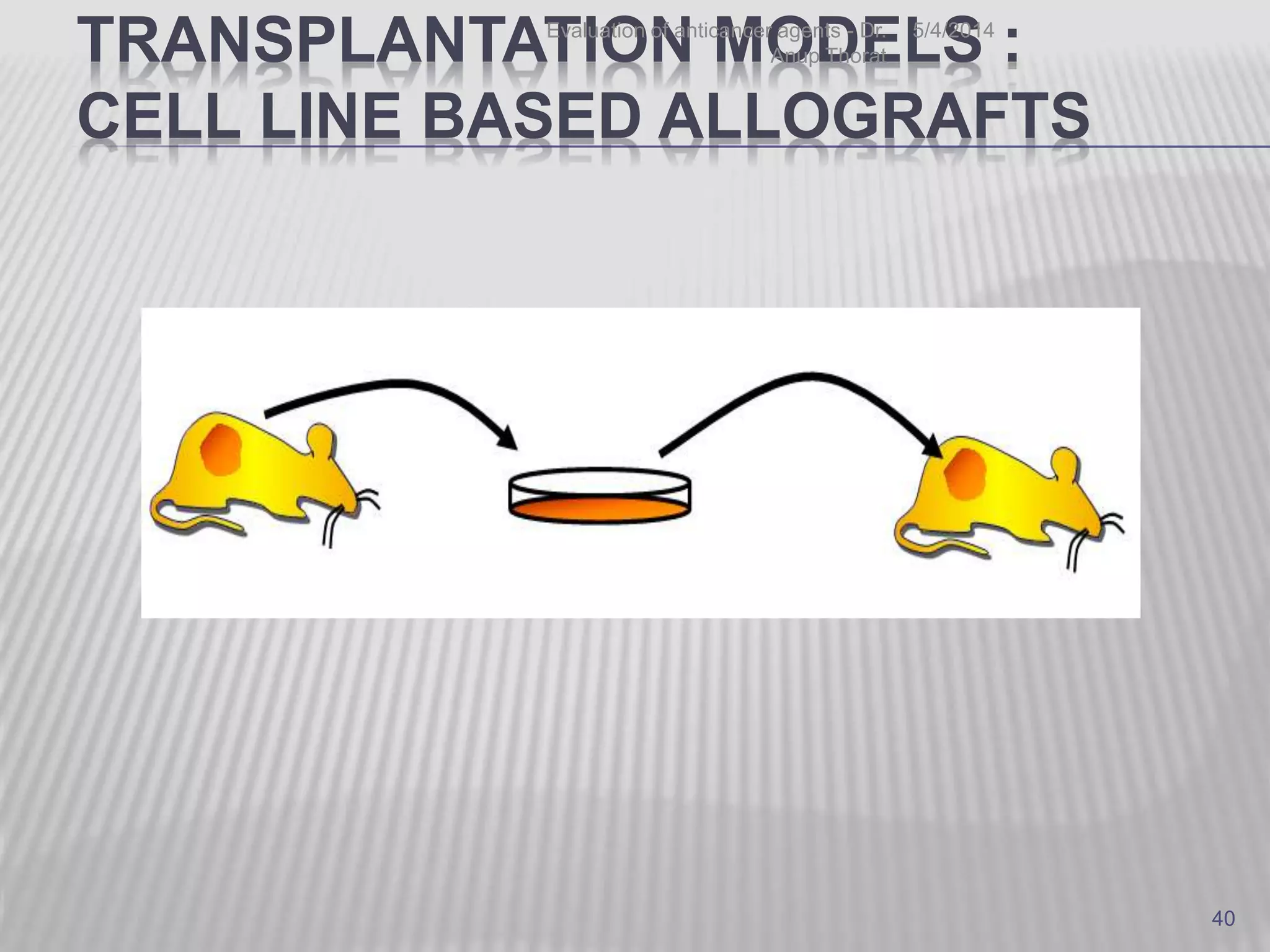
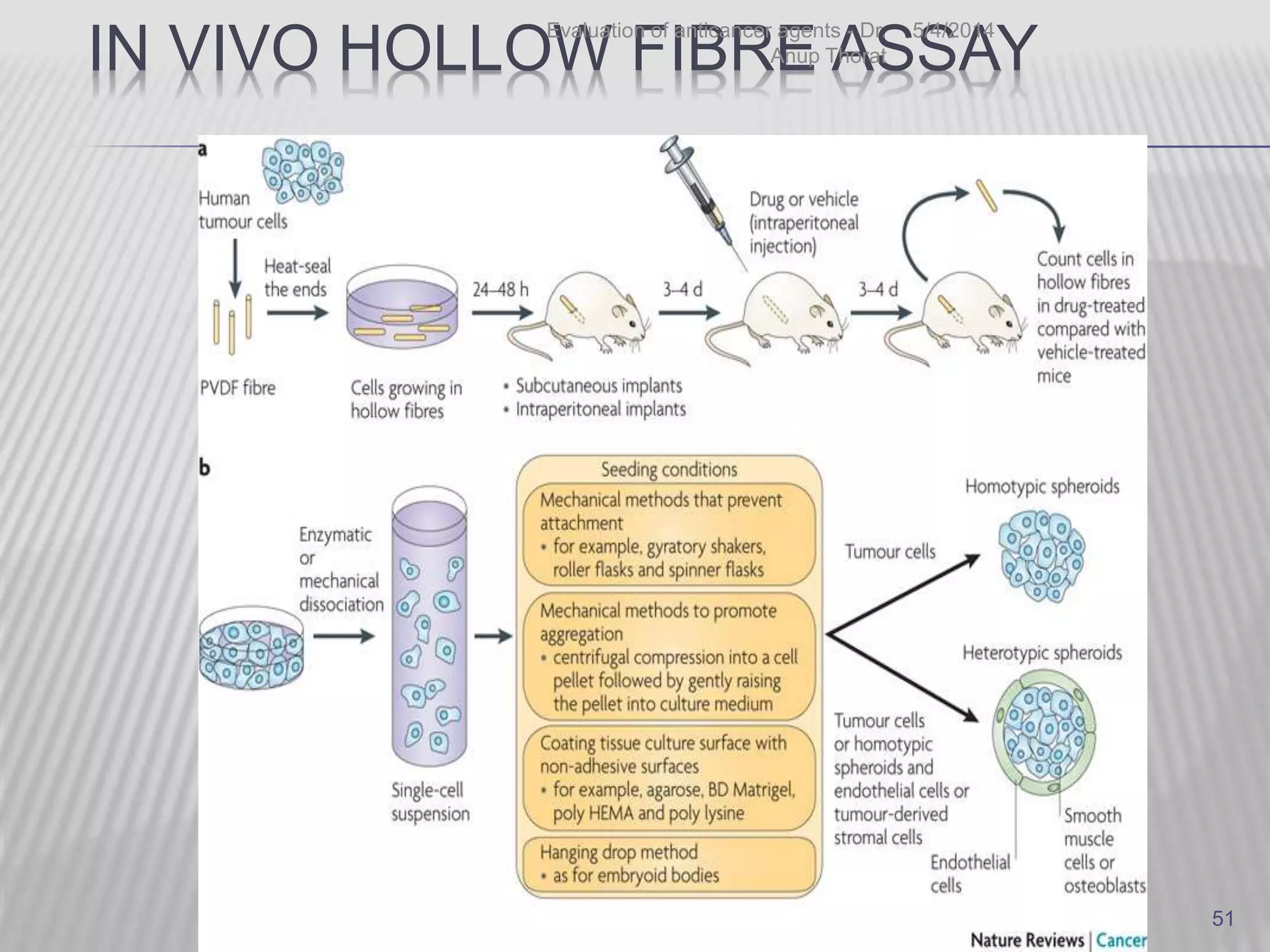
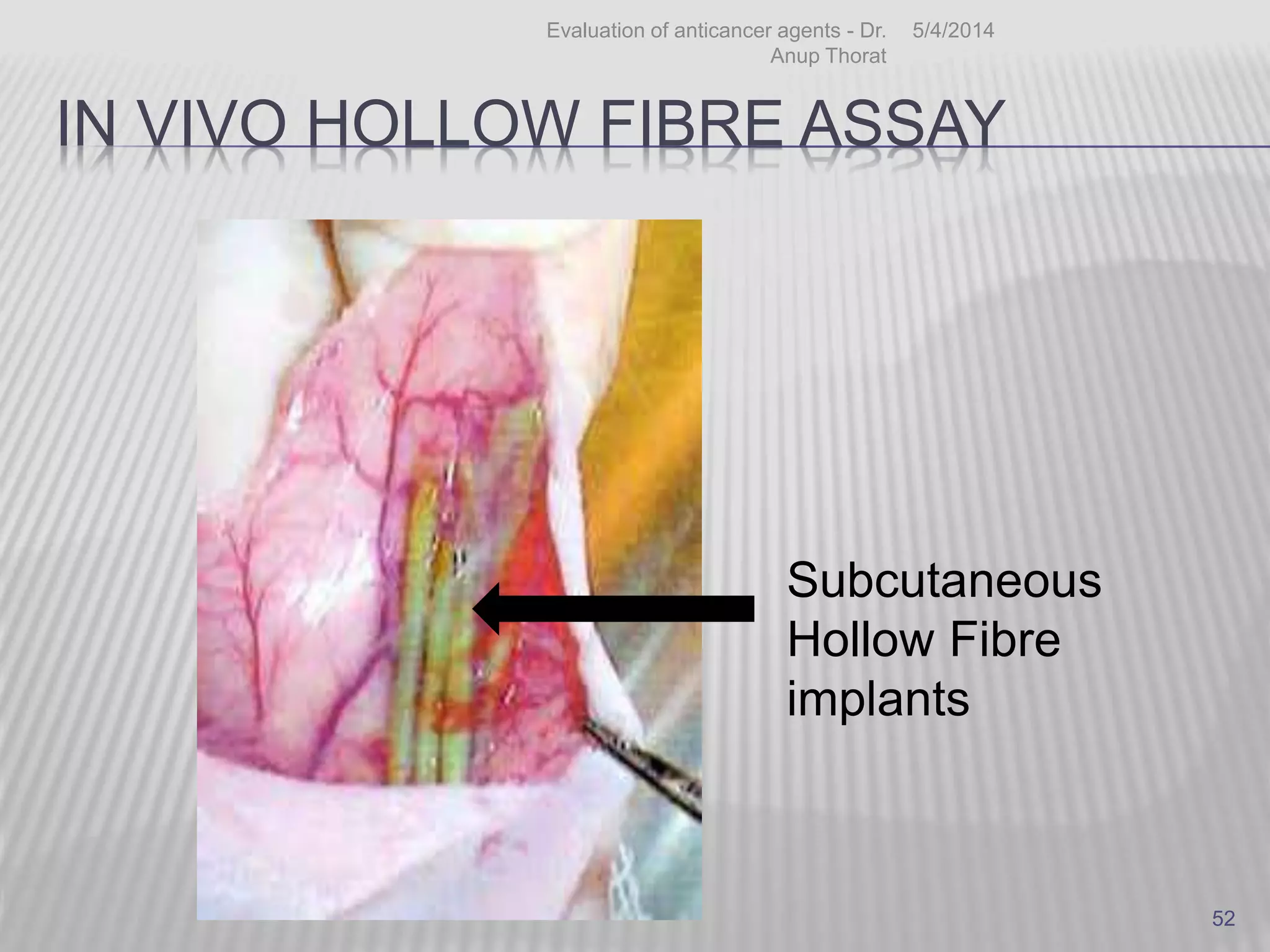
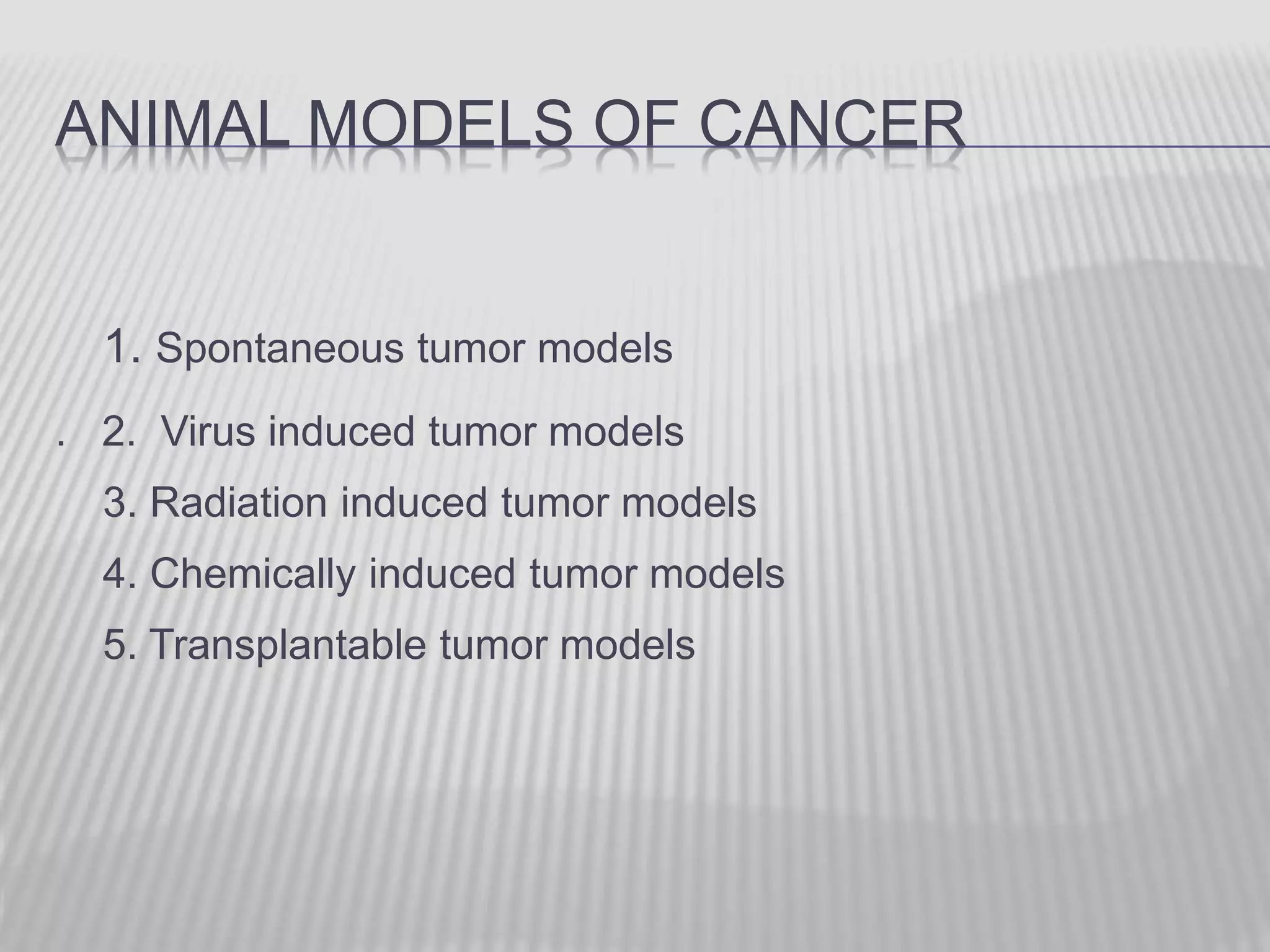
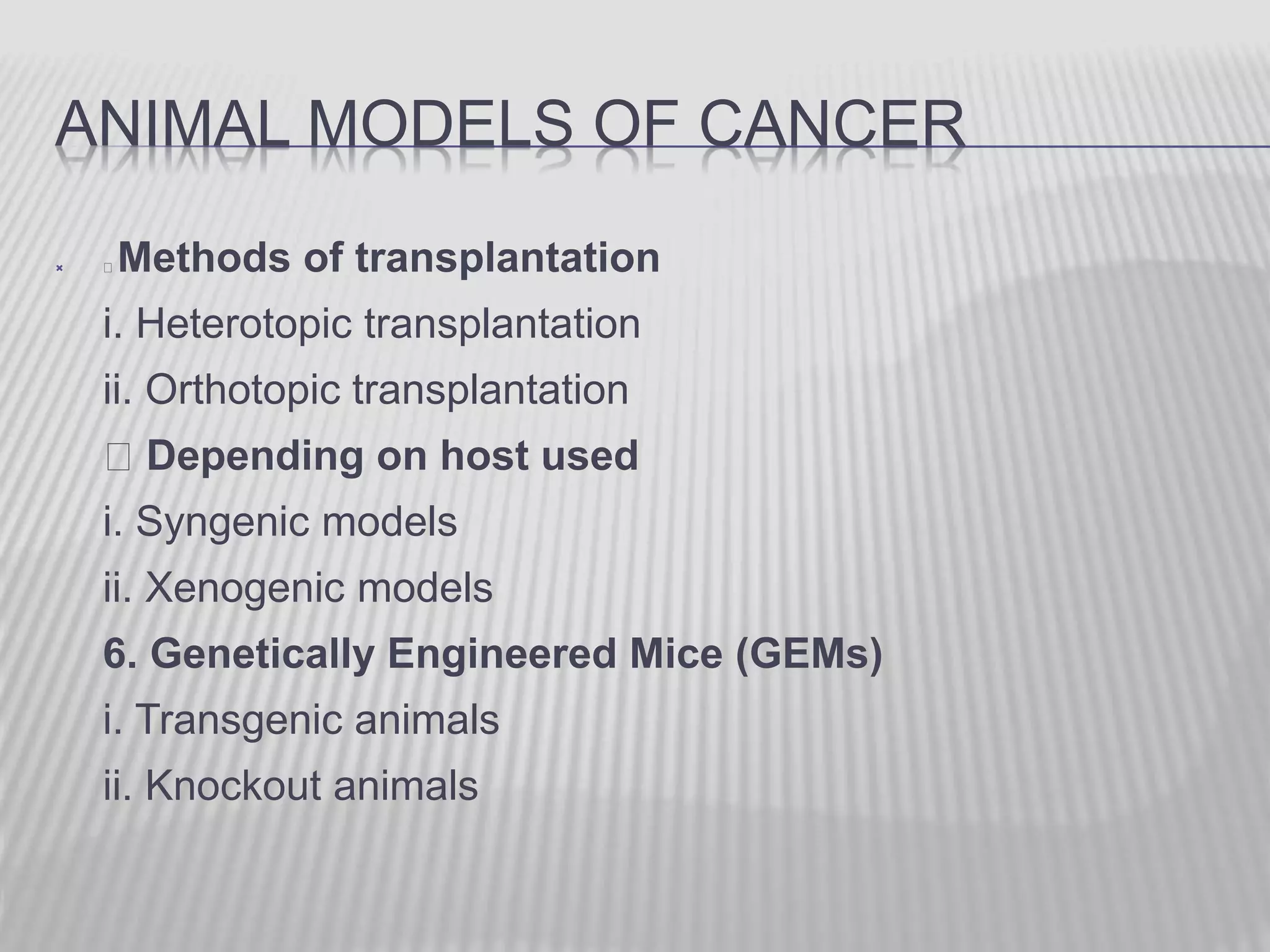
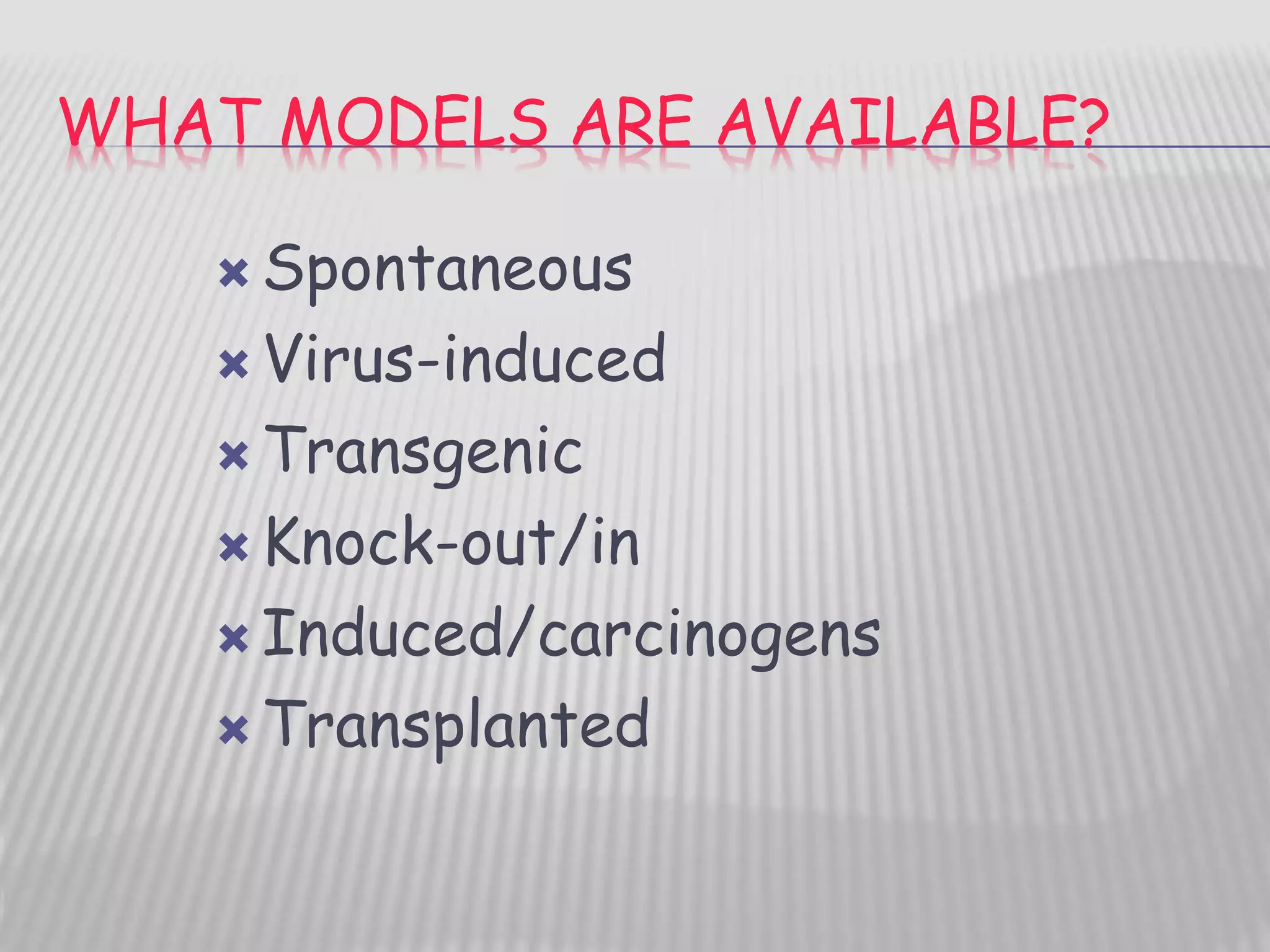
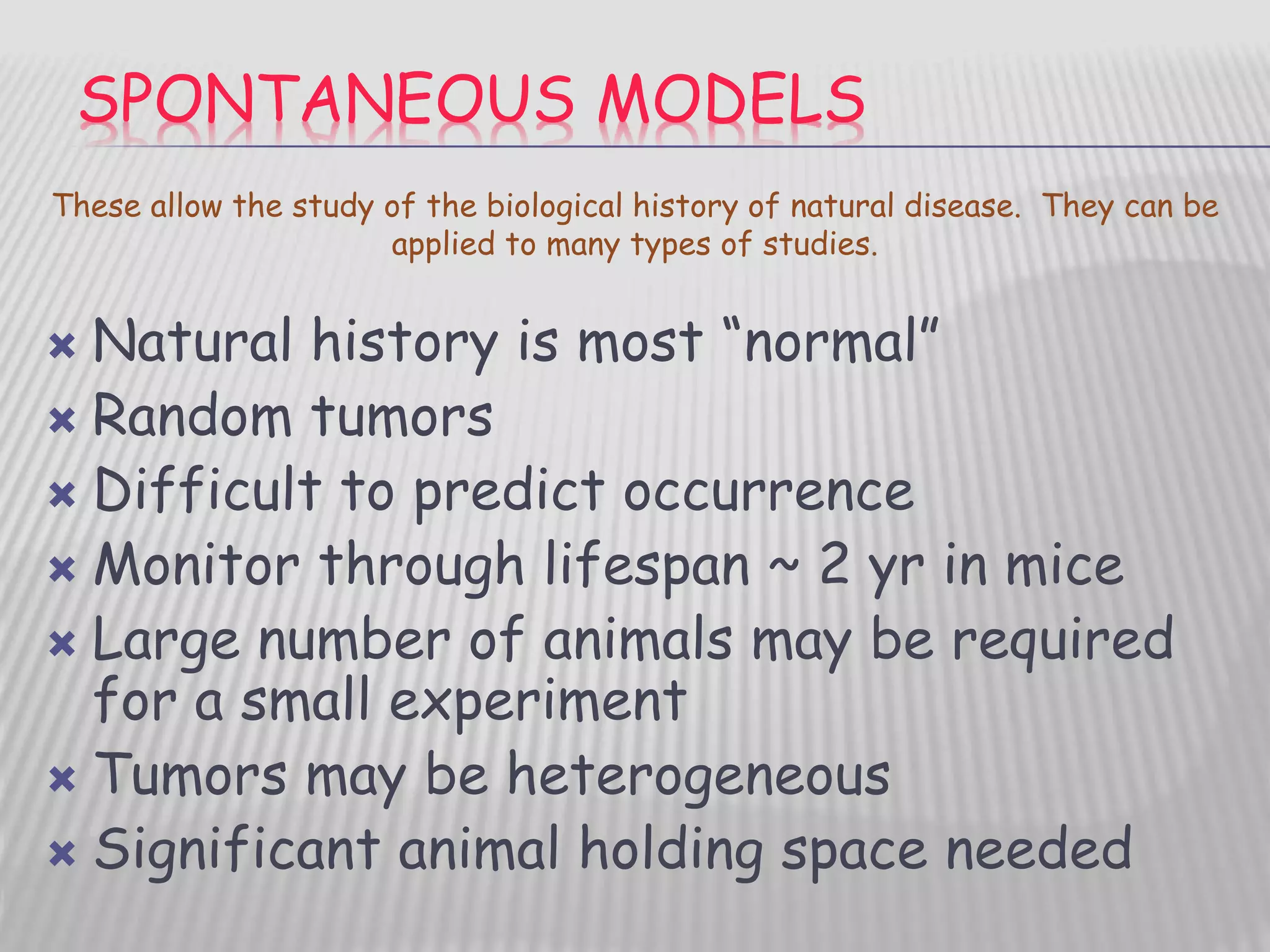
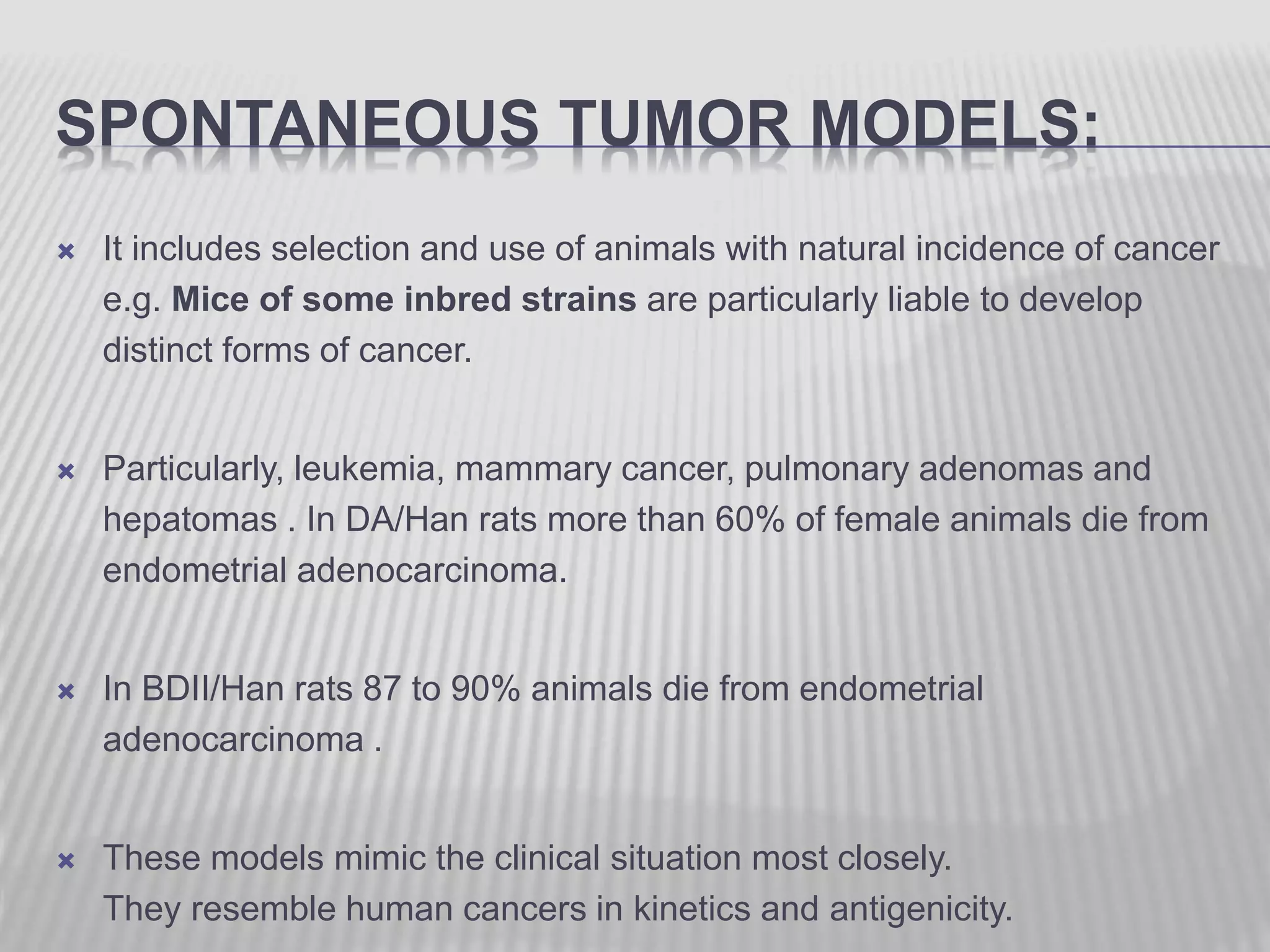
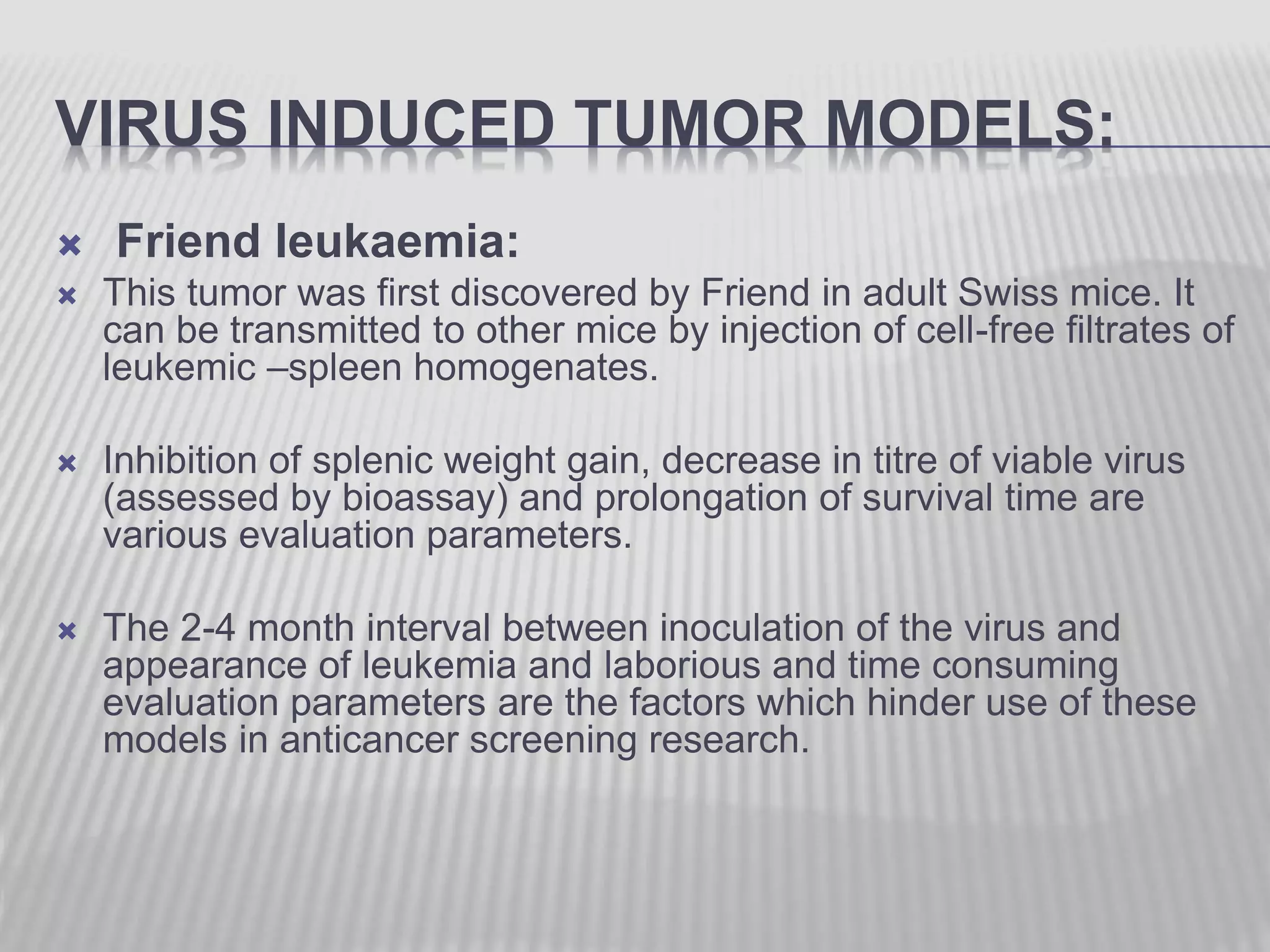
This document discusses various animal models that can be used for cancer drug development and evaluation. It describes several types of models including spontaneous tumor models, virus-induced models, radiation-induced models, chemically-induced models, and transplantable tumor models. Transplantable tumor models involve transplanting cancer cell lines or tissues into mice or rats, which can be done either heterotopically or orthotopically. These models provide advantages such as being easy to control and having many tumor types available, but they do not fully recapitulate human cancer development and progression. The document emphasizes that the appropriate selection of an animal model is crucial for properly evaluating new drug candidates.






![CHEMICAL CARCINOGEN MODEL
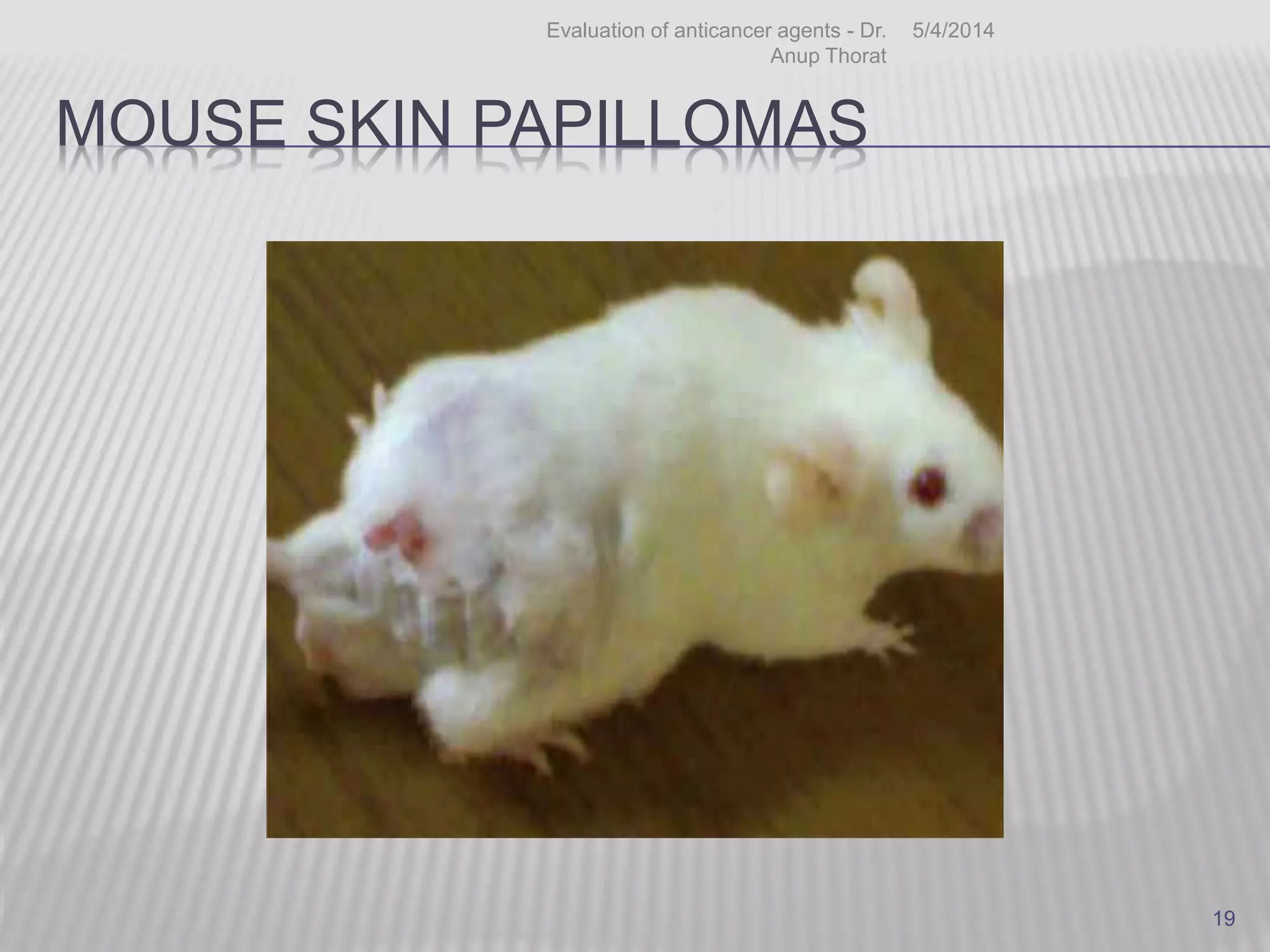
DMBA induced mouse skin papillomas
Two stage experimental carcinogenesis
Initiator – DMBA (dimethylbenz[a]anthracene),
Promotor – TPA. (12-O-tetradecanoyl-phorbol-13-
acetate)
Mice : Single dose – 2.5 µg of DMBA f/b 5 to
10 μg of TPA in 0.2 ml of acetone twice
weekly.
Papilloma begins to appear after 8 to 10 wks
- Tumor incidence & multiplicity of treatment
group is compared with DMBA control group
5/4/2014Evaluation of anticancer agents - Dr.
Anup Thorat
18](https://image.slidesharecdn.com/animalmodelsindevelopmentaltherapeitocs-170923092810/75/Animal-models-in-developmental-therapeitocs-18-2048.jpg)