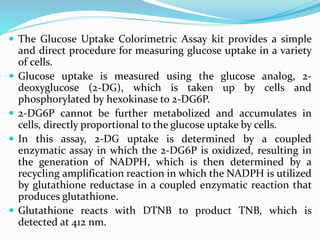
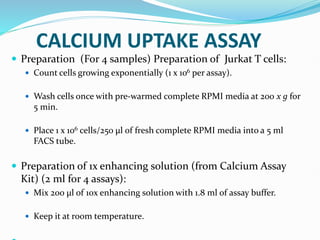
This document provides information on cell culture techniques. It discusses:
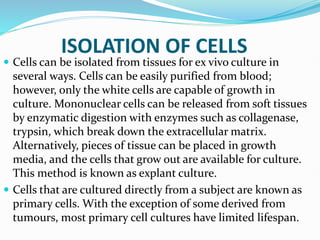
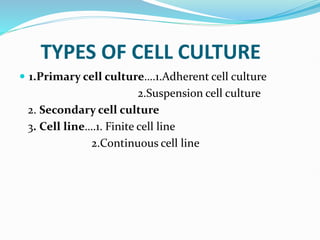
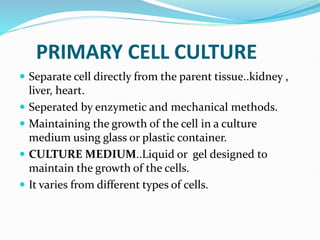
1. Primary cell culture involves directly culturing cells separated from tissues using enzymatic or mechanical methods. Adherent cells attach to surfaces while suspension cells remain floating.
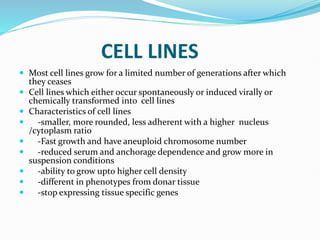
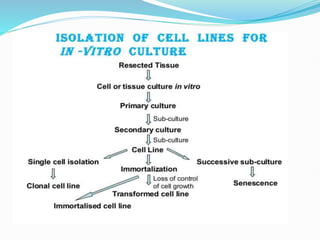
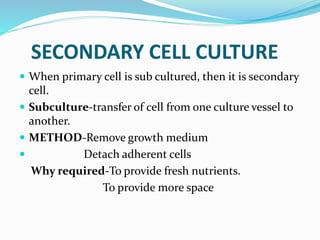
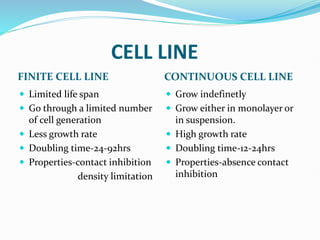
2. Secondary cell cultures are produced when primary cells are subcultured. Cell lines can be either finite, with limited life spans, or continuous, capable of indefinite growth.
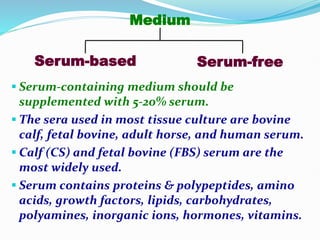
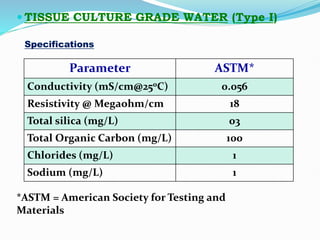
3. Cell culture media must provide nutrients and an appropriate environment for cell growth. Serum-containing and serum-free media are commonly used. Equipment like incubators, storage units, and cryogenic tanks are also needed to maintain cell cultures.









![ Artificial Media
Artificial or synthetic media are prepared by adding
nutrients (both organic and inorganic), vitamins, salts,
O2 and CO2 gas phases, serum proteins, carbohydrates,
cofactors [1].
Different artificial media have been devised to serve one
or more of the following purposes:
1) immediate survival (a balanced salt solution, with
specific pH and osmotic pressure);
2) prolonged survival (a balanced salt solution
supplemented with various formulation of organic
compounds and/or serum);
3) indefinite growth; 4) specialized functions.](https://image.slidesharecdn.com/cellculture-231020075042-c1e43e47/85/Cell-Culture-pptx-17-320.jpg)