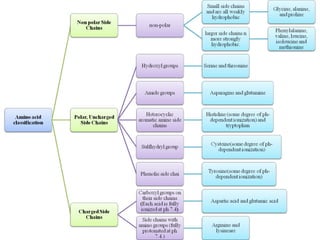
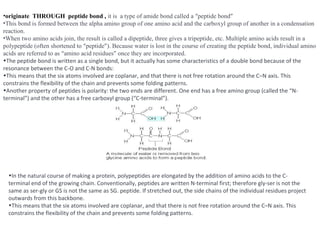
Amino acids are the building blocks of proteins. They contain both a carboxyl and amino group attached to the same carbon atom. Although commonly depicted as neutral molecules, amino acids exist as zwitterions with both positive and negative charges.
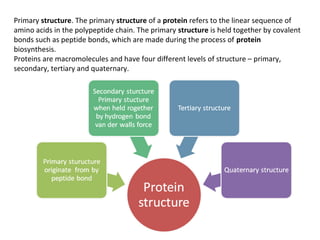
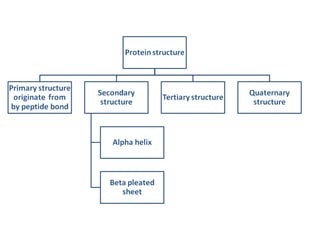
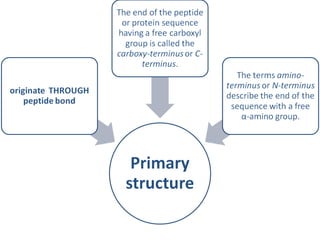
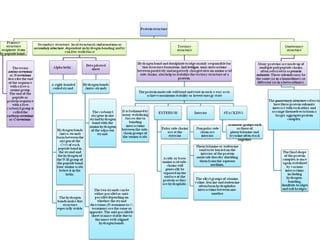
The primary structure of a protein refers to the linear sequence of amino acids in its polypeptide chain. Amino acids are joined by peptide bonds formed via a condensation reaction between the amino group of one amino acid and the carboxyl group of the next. This results in a chain of amino acid residues with different properties at each end.