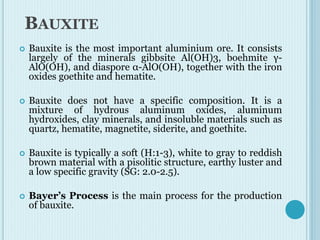
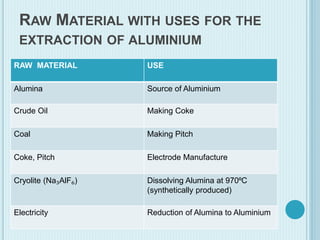
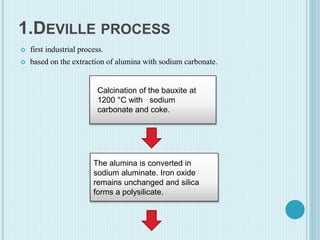
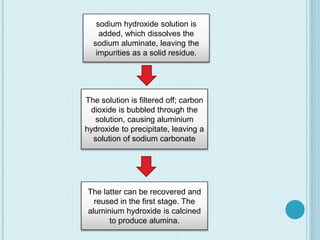
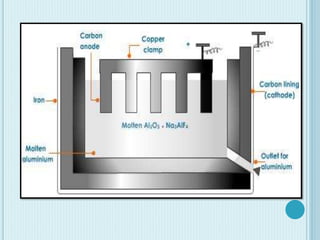
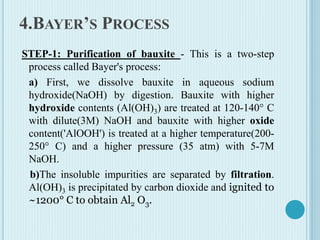
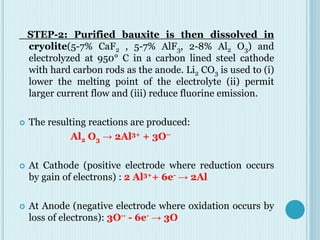
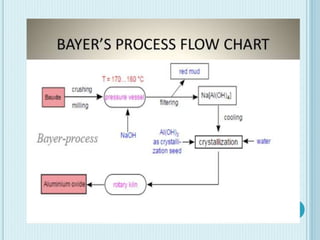
Aluminum is a lightweight metal that is widely used due to its properties and extraction process. It can be extracted from bauxite ore through the Bayer process, which involves dissolving the aluminum-containing minerals in sodium hydroxide to produce alumina, which is then electrolyzed to produce aluminum metal. Aluminum is commonly used in alloys to improve strength and is applied in transportation and construction due to its corrosion resistance, electrical conductivity, and high strength to weight ratio.


![BASIC INFORMATION :
Atomic number ?
Electronic configuration?
Density ?
Atomic mass?
Melting point?
Boiling point ?
13
[Ne]3s23p1
2.7 g/cm3
26.9g
2519∘C
660∘C](https://image.slidesharecdn.com/nfmtaluminium-180503182946/85/aluminium-extraction-5-320.jpg)

![Humphry Davy : that aluminium could be
produced by electrolytic reduction from
alumina (aluminium oxide).
Hans Christian Oersted (Denmark) :
Was successful in extracting but produced an aluminium
alloy rather than pure aluminium.
Friedrich Woehler [German] :
continued Hans Christian’s work.
1808
1825
1827
1846
Friedrich created small balls of
solidified molten aluminium (globules)
1856 Henri-Etienne Sainte-Claire Deville[French] :
industrial applications. [DEVILLE PROCESS]
1856-1890
200 tonnes
of Al were produced in 36 years](https://image.slidesharecdn.com/nfmtaluminium-180503182946/85/aluminium-extraction-7-320.jpg)