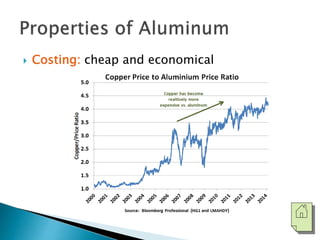
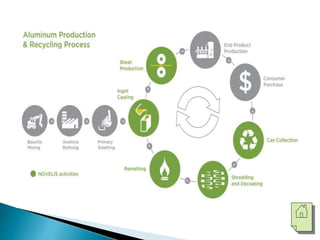
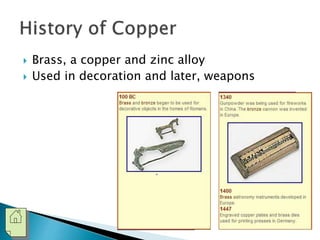
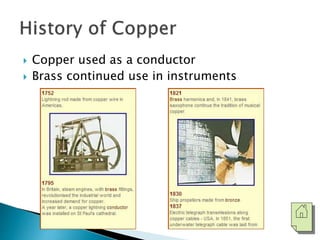
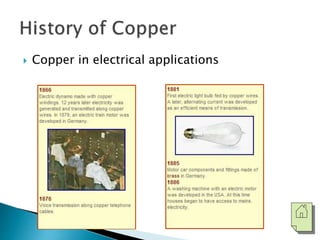
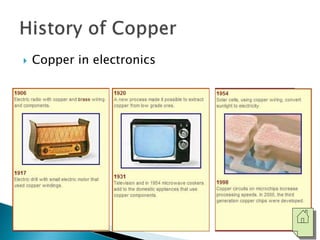
The document discusses the properties, processing, and applications of aluminum and copper. Aluminum is lightweight, strong, and highly recyclable, used in various industries including aerospace, automotive, and packaging, while copper is known for its excellent electrical and thermal conductivity, utilized in electrical applications and plumbing. Both metals undergo extensive processing from ore extraction to refining for use in myriad applications.