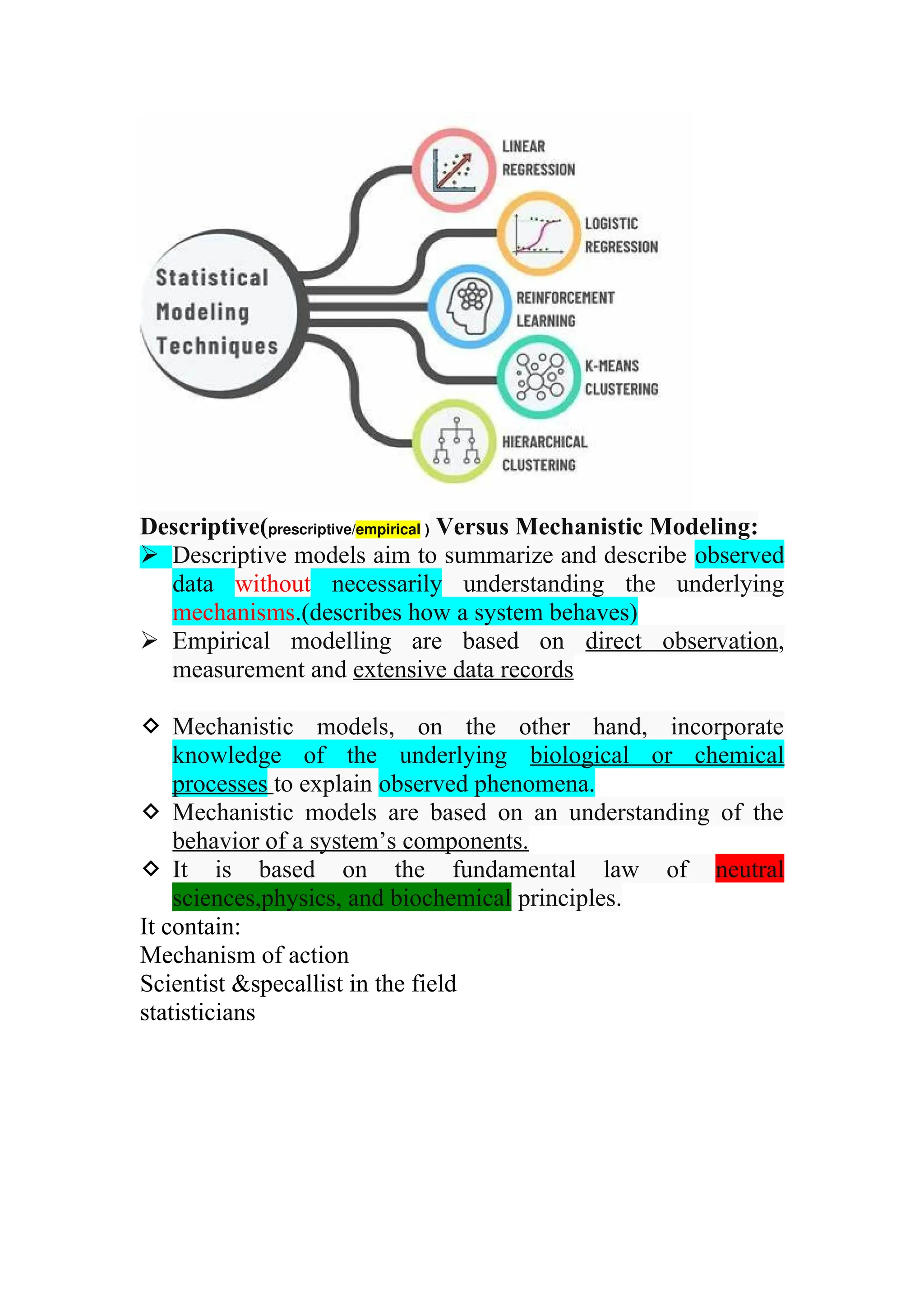
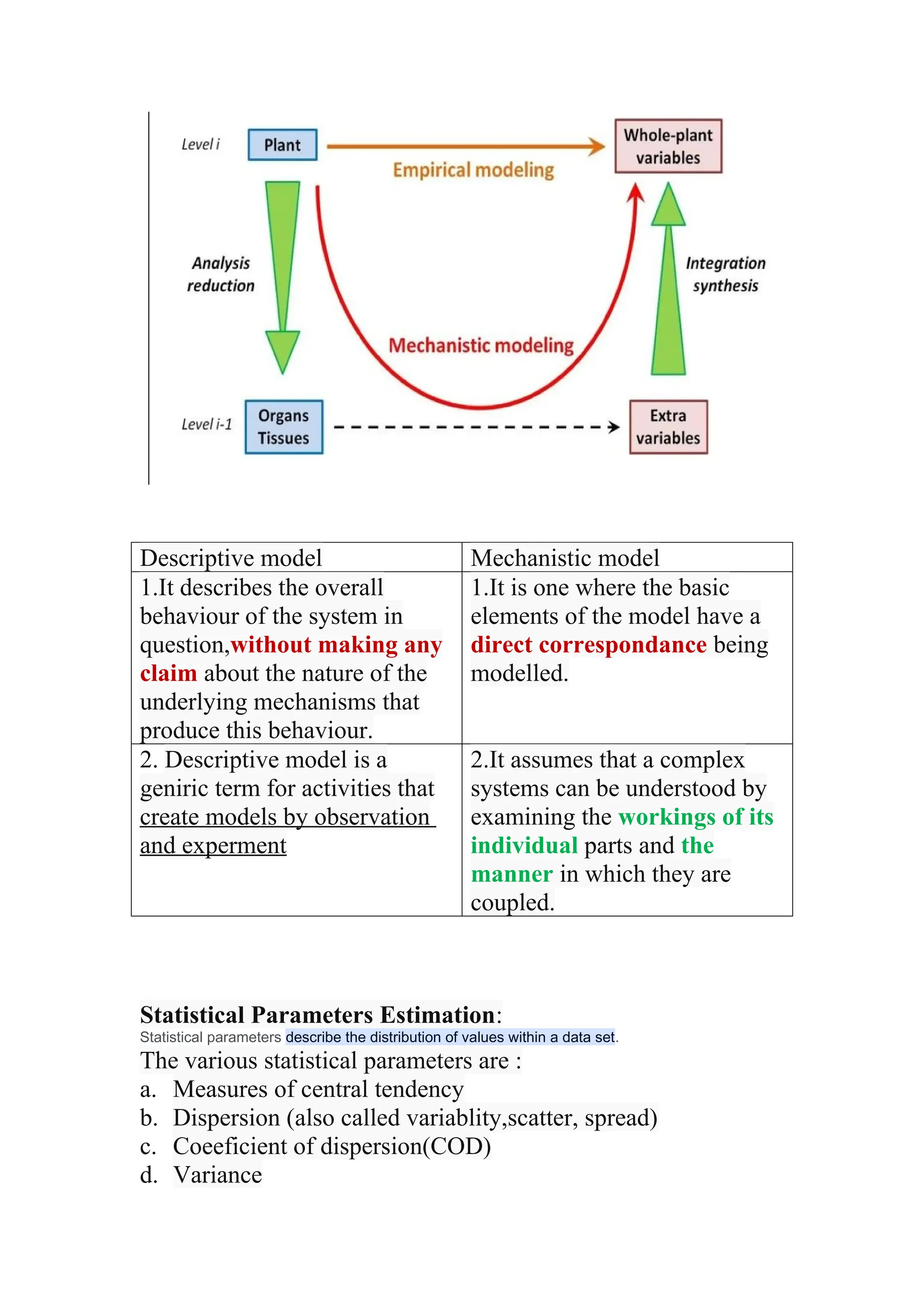
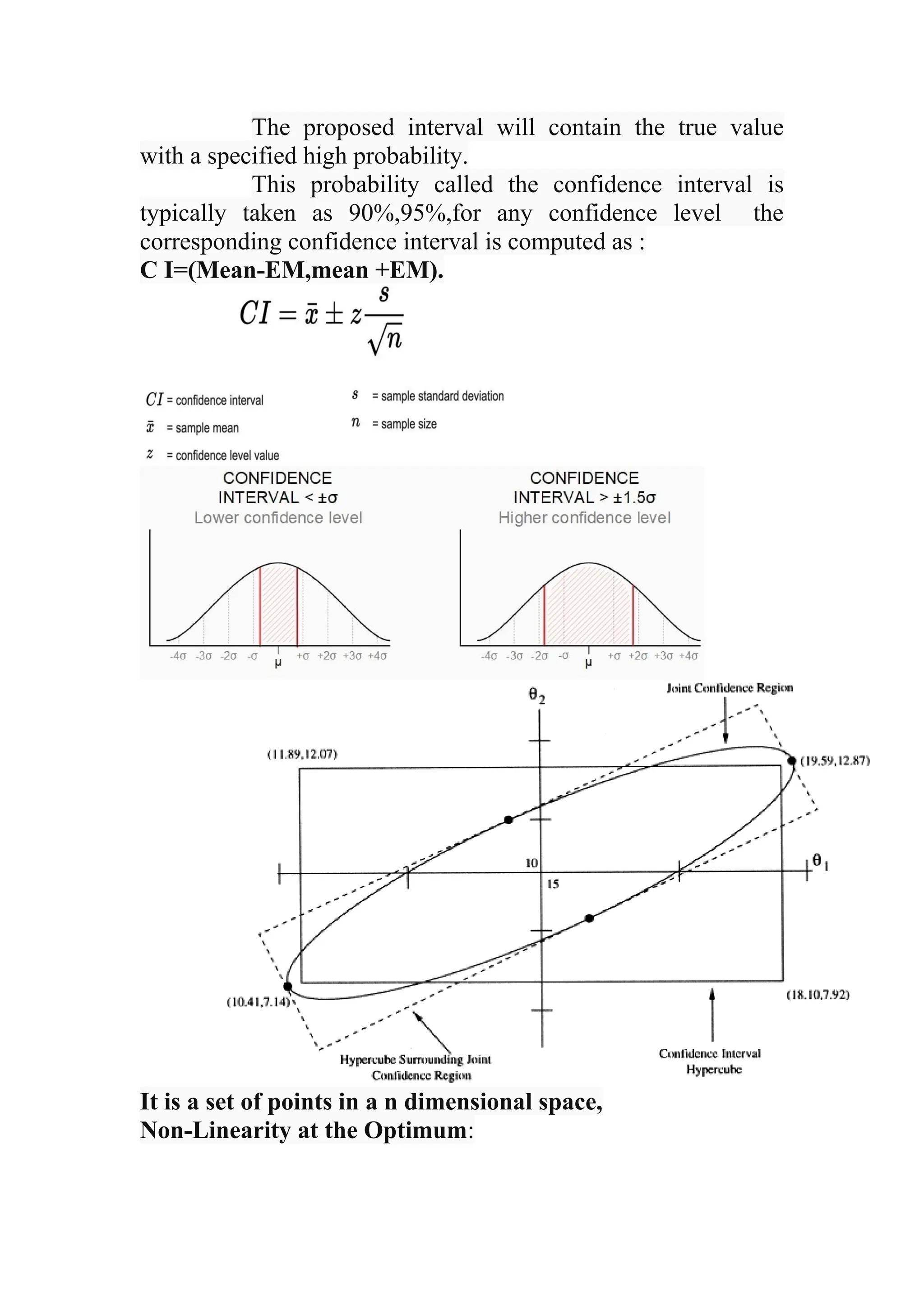
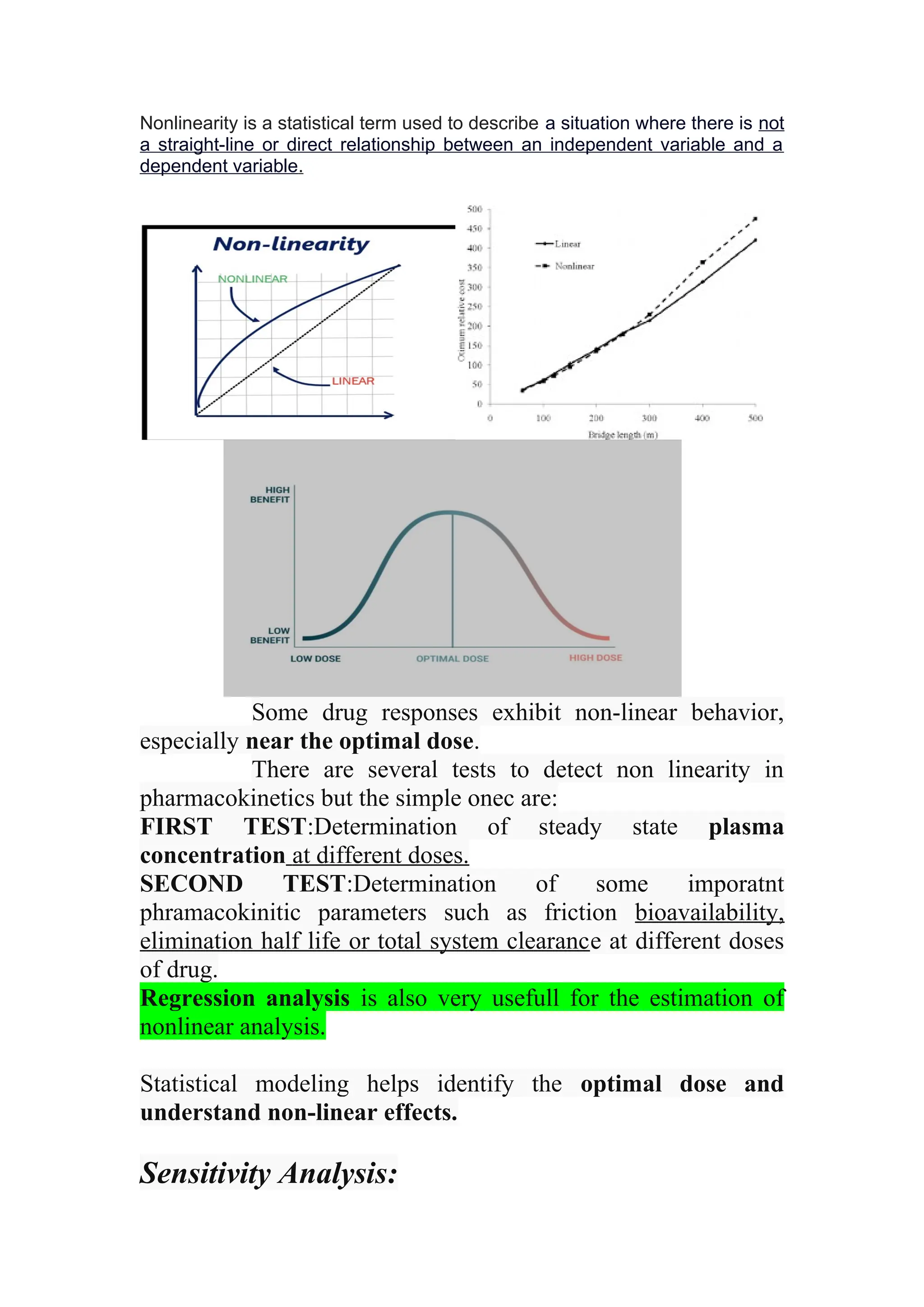
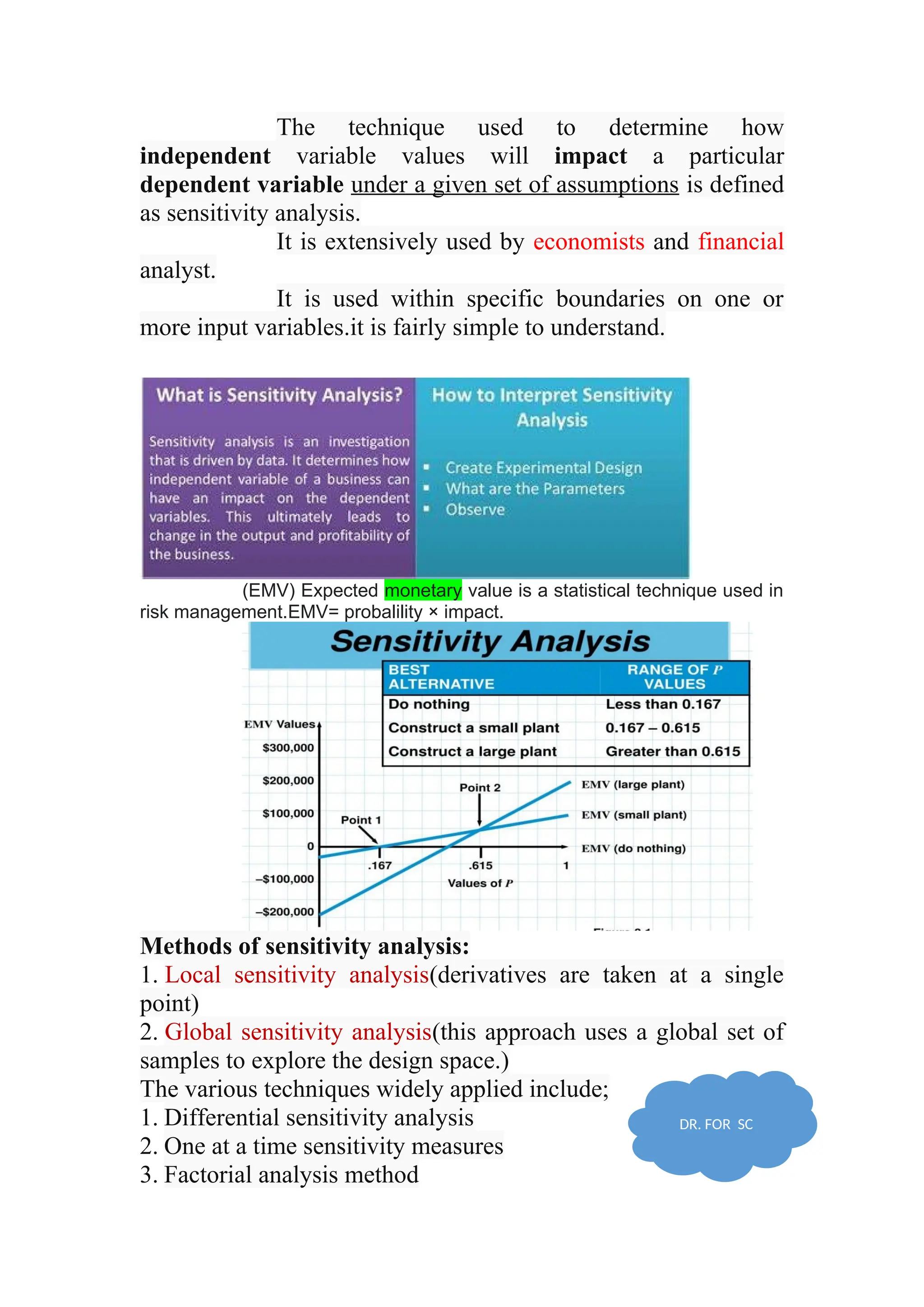
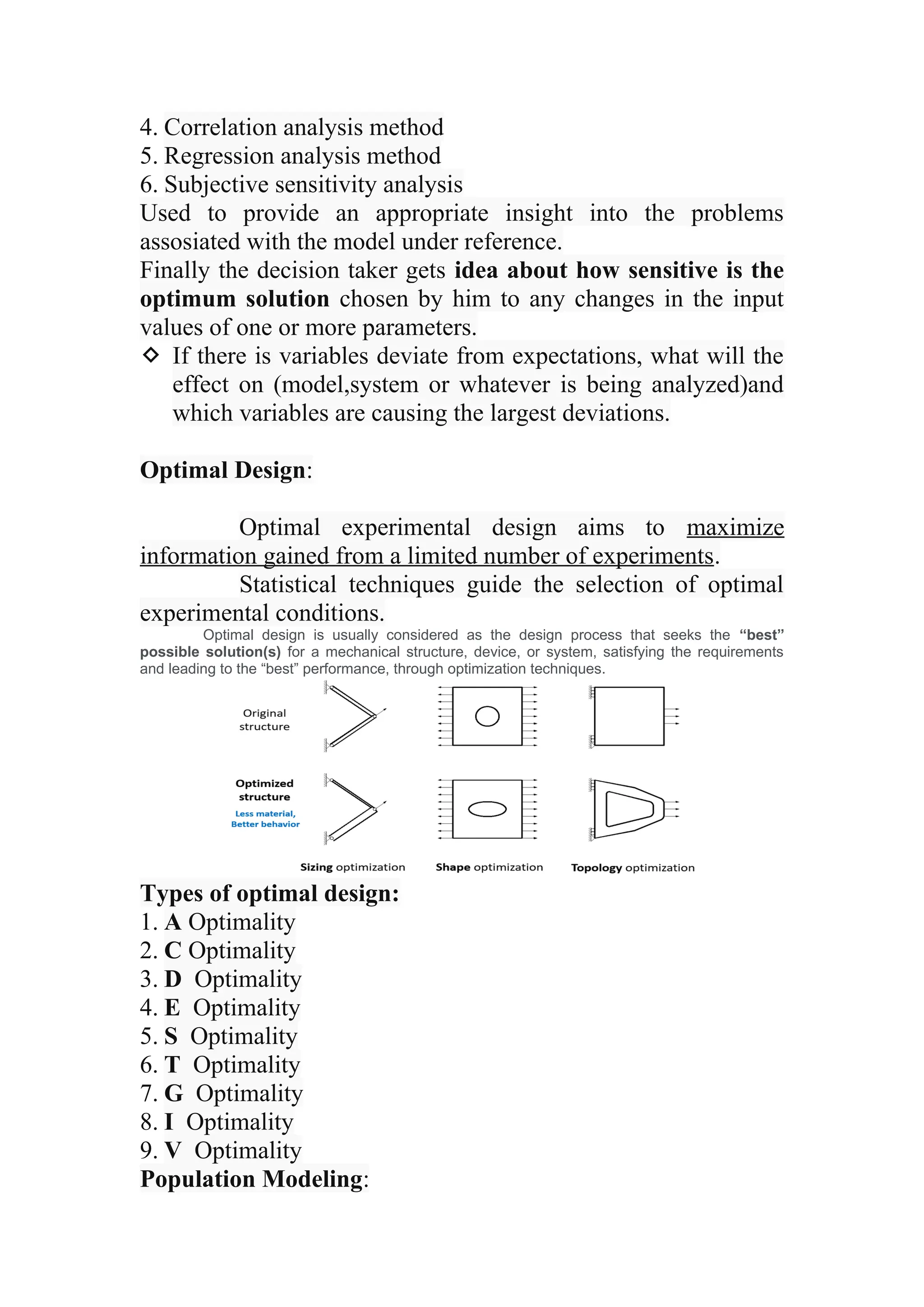
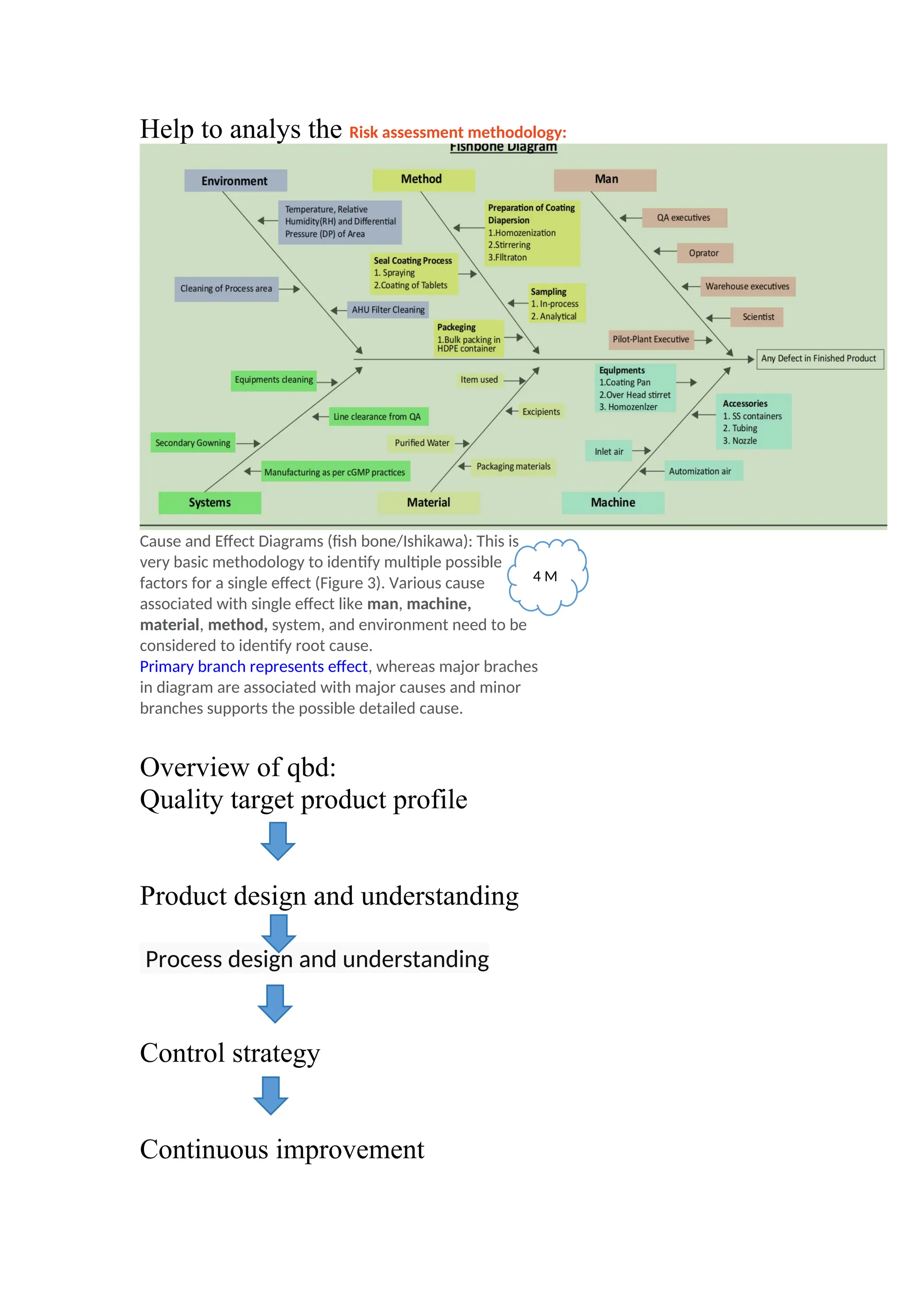
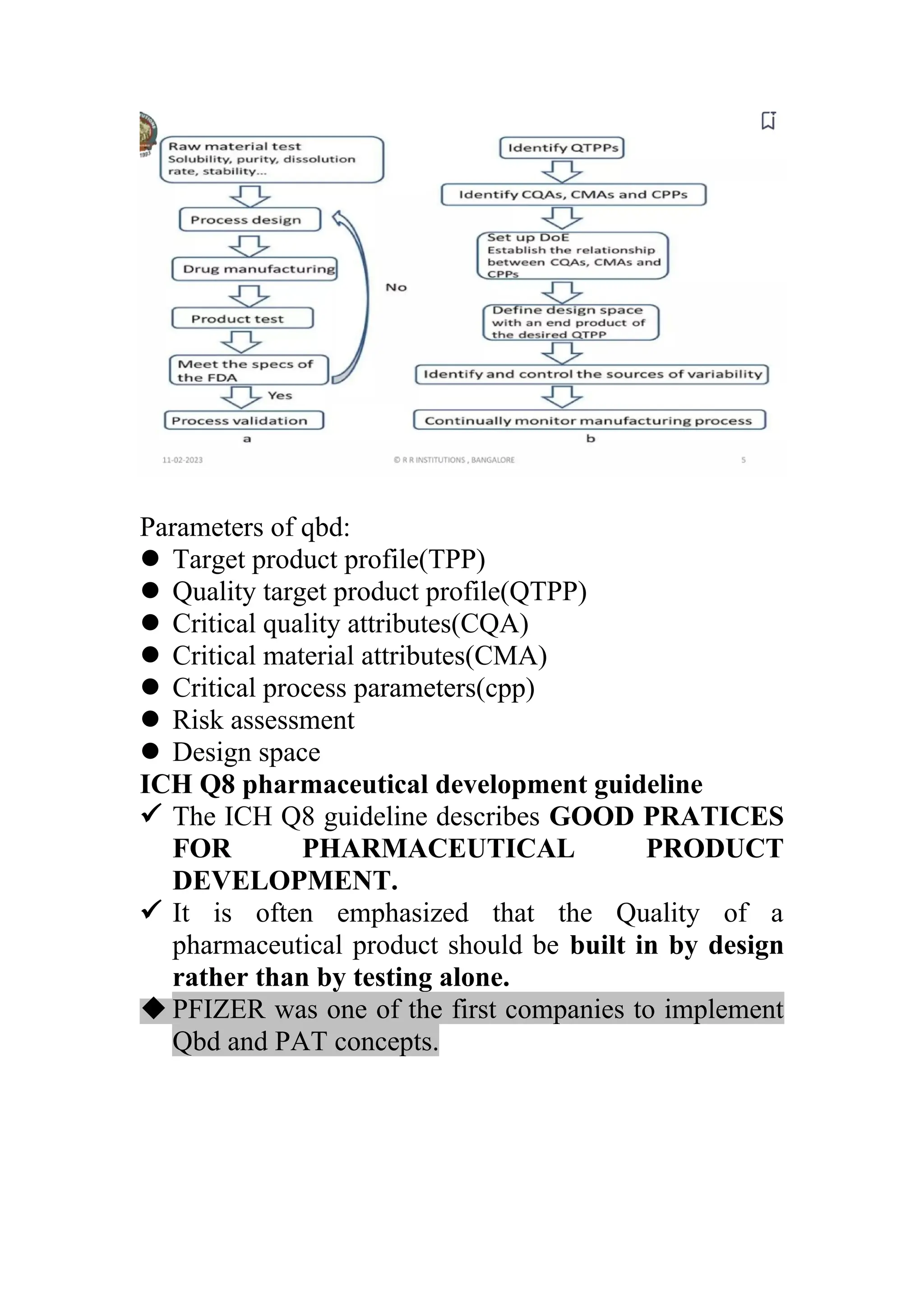
The document discusses computer-aided drug design using statistical modeling, explaining the differences between descriptive and mechanistic modeling, as well as their applications in predicting drug behavior and efficacy. It covers statistical parameter estimation, sensitivity analysis, and optimal experimental design while emphasizing quality by design (QbD) principles and their importance in pharmaceutical development. The QbD approach aims to enhance product quality through systematic understanding and continuous improvement in drug manufacturing processes.