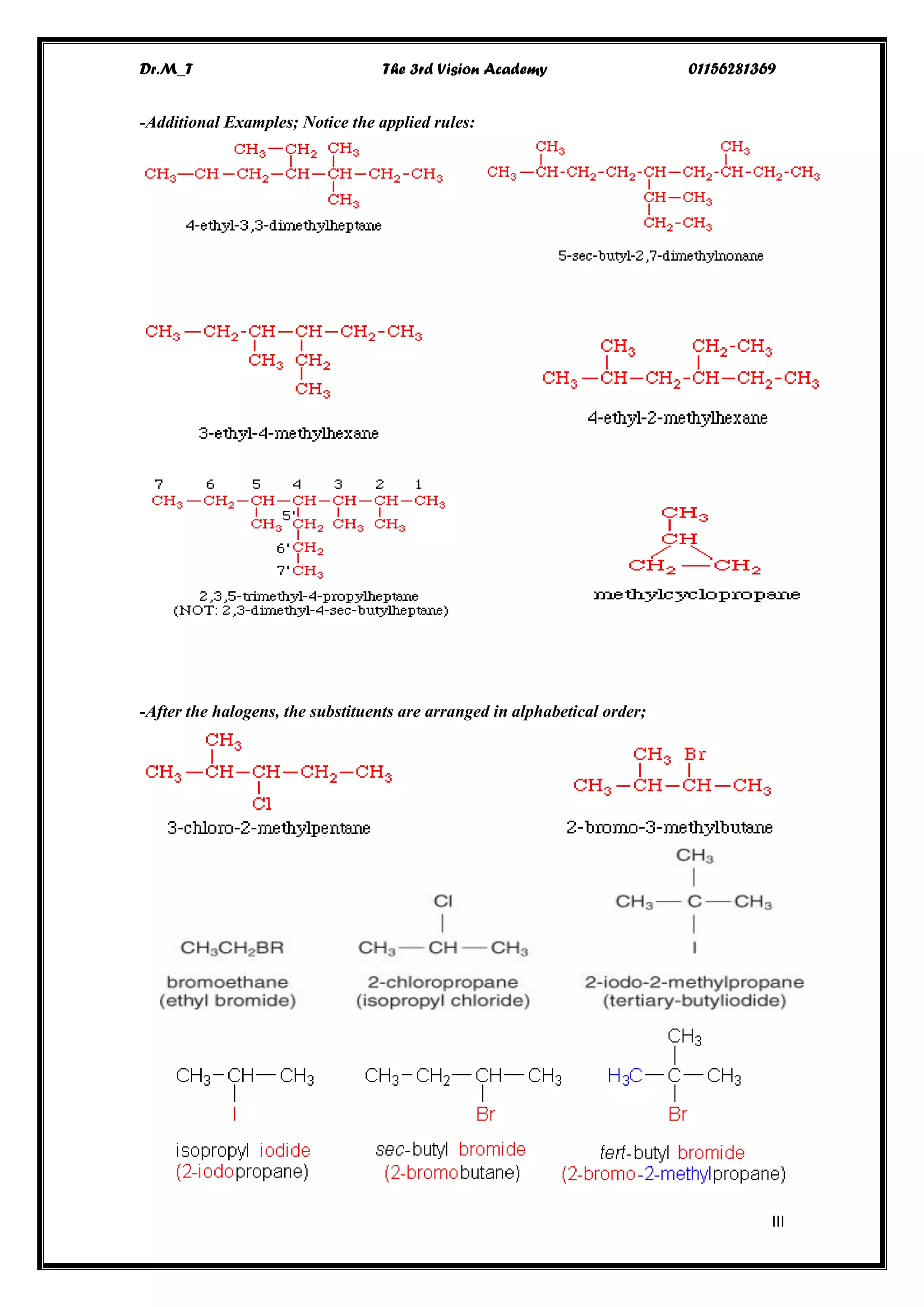
The document details the IUPAC nomenclature system for organic compounds, focusing specifically on alkanes, which are hydrocarbons composed solely of carbon and hydrogen with single bonds. It outlines the fundamental principles and rules for naming alkanes, including identifying the longest carbon chain and the proper ordering of substituents. Additionally, it covers the physical properties, reactivity, and various reactions involving alkanes.