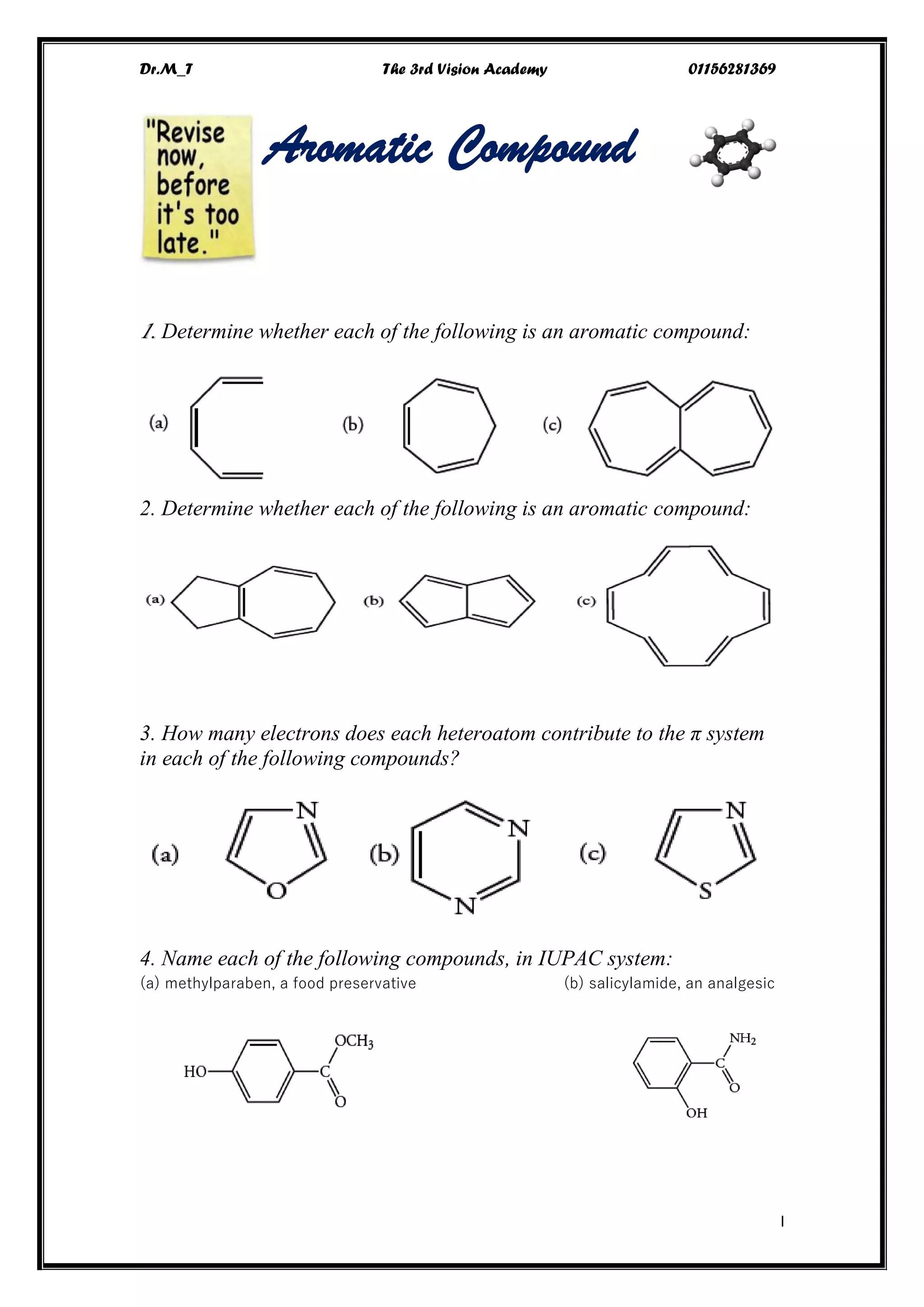
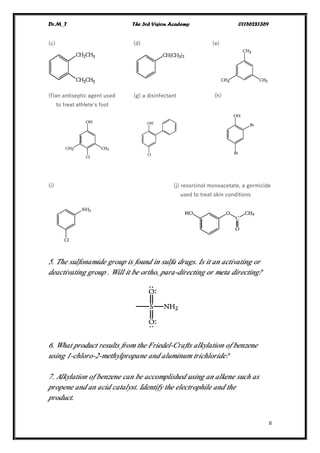
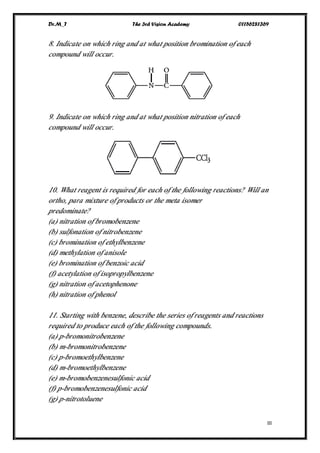
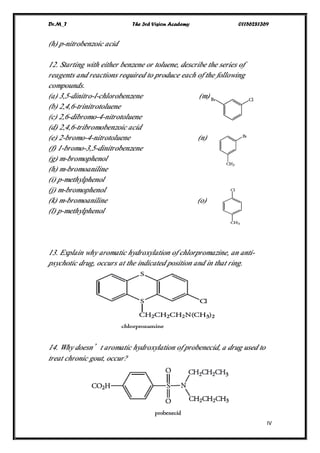
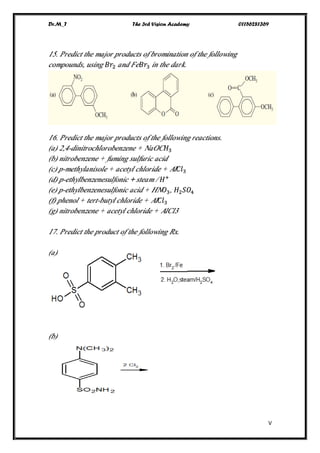
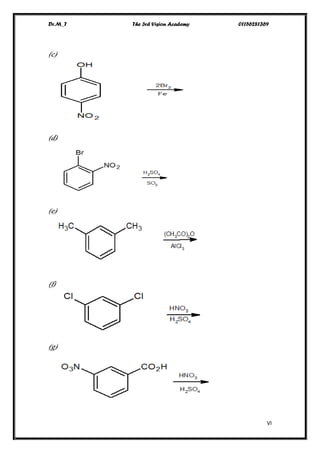
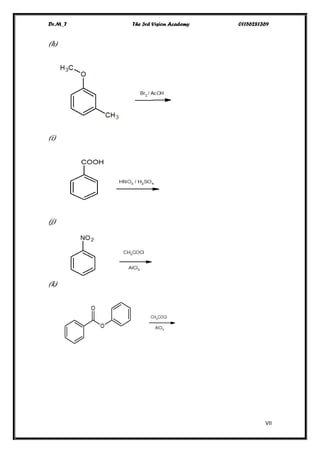
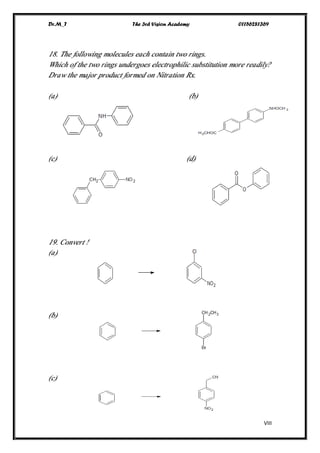
This document contains a series of questions about aromatic compounds and electrophilic aromatic substitution reactions. It asks the reader to identify aromatic compounds, predict products of various substitution reactions involving benzene and related aromatic rings, explain directing effects of different substituents, and draw resonance structures of arenium ions formed in electrophilic aromatic substitution. The questions cover topics such as naming compounds, determining substituent effects, predicting reaction mechanisms, and requiring multi-step synthesis of aromatic compounds.