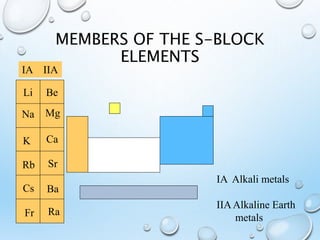
The s-block elements include the alkali metals (Group 1) and alkaline earth metals (Group 2). Some key properties include:
1) They have the lowest first ionization energies in each period and metallic character increases down each group as it becomes easier to lose electrons.
2) They form ionic compounds with fixed 1+ oxidation states for alkali metals and 2+ for alkaline earth metals.
3) Their oxides and hydroxides are basic and increase in basic strength down each group.
4) They react vigorously with water to form hydroxides and hydrogen gas. Reactivity increases down each group.






















![s-block-elements [Autosaved].ppt](https://image.slidesharecdn.com/s-block-elementsautosaved-230605165934-44b5daea/85/s-block-elements-Autosaved-ppt-24-320.jpg)
![s-block-elements [Autosaved].ppt](https://image.slidesharecdn.com/s-block-elementsautosaved-230605165934-44b5daea/85/s-block-elements-Autosaved-ppt-25-320.jpg)