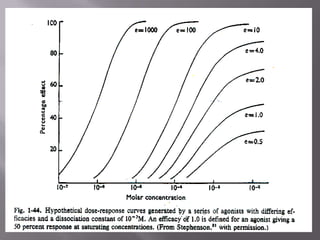
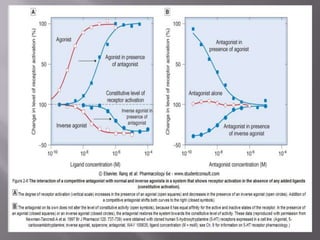
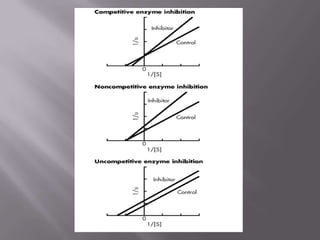
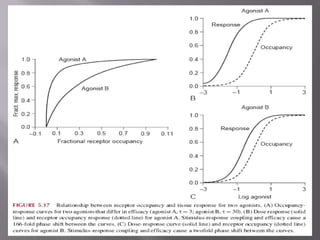
The document discusses key concepts related to how ligands bind to proteins and receptors. It defines important terms like:
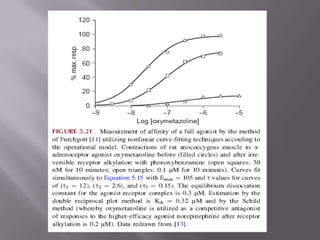
1) Equilibrium dissociation constant (Keq), which represents the concentration of ligand that occupies 50% of receptor sites. Keq is inversely related to affinity.
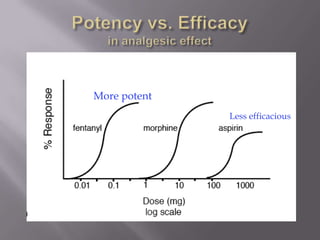
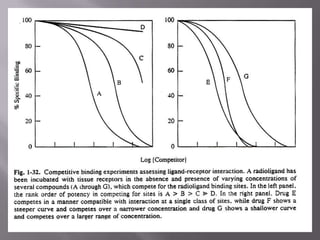
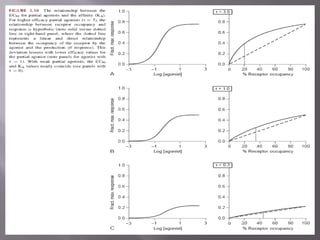
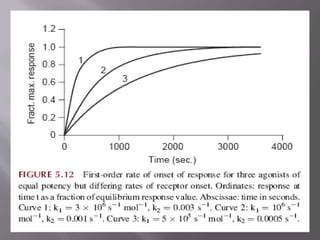
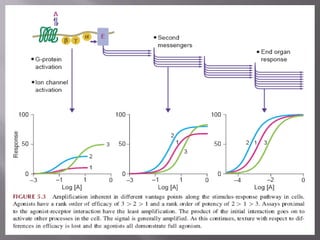
2) Potency, which refers to the concentration of a drug needed to produce a given effect. It is determined by receptor affinity.
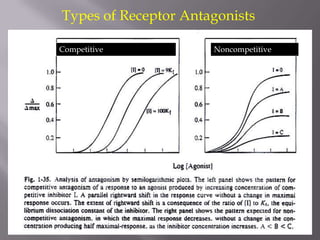
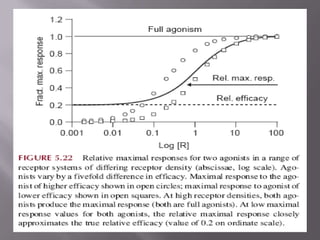
3) Efficacy, which represents a drug's ability to induce a physiological response through a receptor. Full agonists elicit the maximum response while partial agonists have lower efficacy.










![PARTIAL AGONISTS – EFFICACY
Even though drugs may occupy the same of receptors, the magnitude of their
effects may differ.
Full Agonist
1.0
Partial agonist
0.8
% Maximal Effect
0.6 Partial agonist
0.4
0.2
0.0
0.01 0.10 1.00 10.00 100.00 1000.00
[D] (concentration units)](https://image.slidesharecdn.com/affinityandefficacy-120913153600-phpapp02/85/Affinity-and-Efficacy-16-320.jpg)


![Measuring Drug Action
Efficacy and Potency
More
potent
than D2
D1 D2
100
% Response D3
Less
efficacious
than D2
0
Log [Drug]
…… Potency corresponds to the strength of a drug, while Efficacy corresponds to the
effectiveness of a drug.
…… e.g., if 5 mg of drug A relieves pain as effectively as 10 mg of drug B, drug A
is twice as potent as drug B
…… the diuretic furosemide eliminates much more salt and water through urine than
does the diuretic chlorothiazide. Thus, furosemide has greater efficacy than
chlorothiazide.
MEDC 603 Fall 2007](https://image.slidesharecdn.com/affinityandefficacy-120913153600-phpapp02/85/Affinity-and-Efficacy-19-320.jpg)
![Measuring Drug Action
Efficacy / Potency / Toxicity
Desired Response (%)
100 100
Toxicity (%)
50 50
0 0
Log [Drug]
Blue lines …. Drug 1
Red lines …. Drug 2
Solid lines …. Desired response
Dotted lines …. Toxicity response
MEDC 603 Fall 2007](https://image.slidesharecdn.com/affinityandefficacy-120913153600-phpapp02/85/Affinity-and-Efficacy-20-320.jpg)