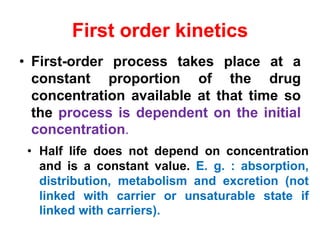
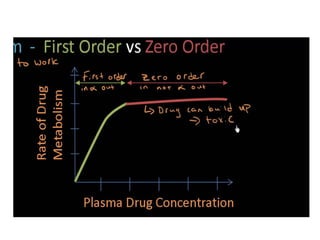
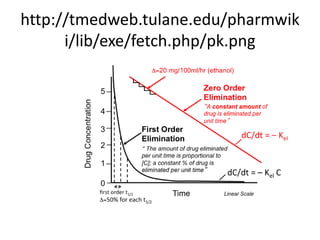
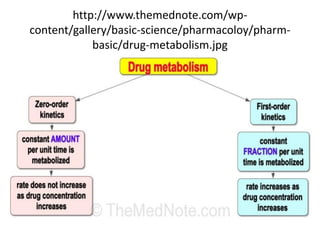
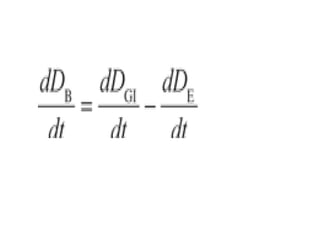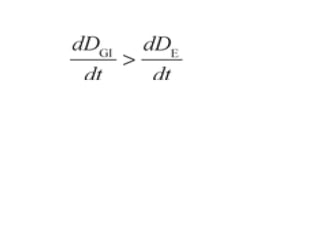The document discusses pharmacokinetics, focusing on zero and first-order processes in drug absorption and elimination. It explains how the rate of drug absorption depends on various factors and distinguishes between the two kinetics, highlighting that most drugs exhibit first-order kinetics under typical conditions, while zero-order kinetics can occur in saturated situations. Key examples are provided, such as IV infusions demonstrating zero-order kinetics and common drugs eliminated by first-order kinetics.