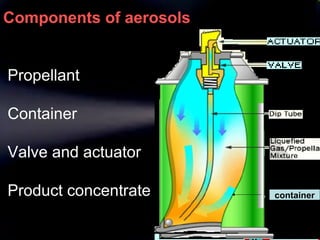
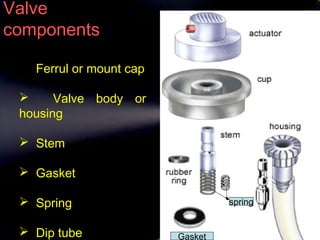
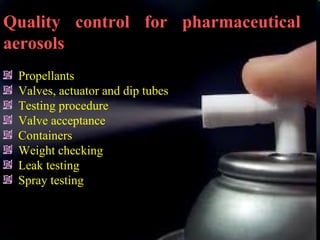
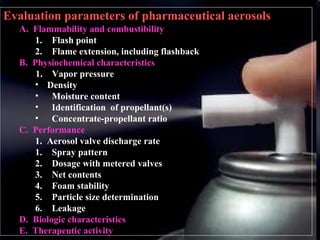
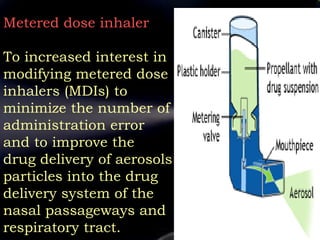
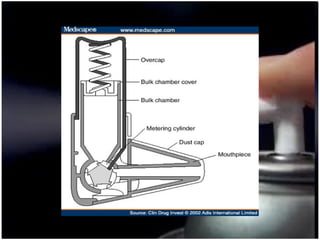
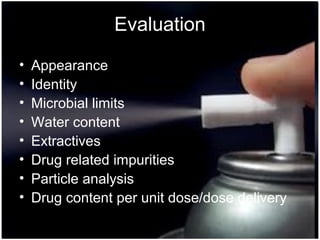
Pharmaceutical aerosols are therapeutic active ingredients packaged in a pressurized system. They have advantages like direct delivery to affected areas and reduced irritation. Components include a propellant, container, valve, and product concentrate. Propellants expel the product and include hydrocarbons or gases. Containers must withstand high pressure. Valves deliver the drug in the desired form. Formulations contain an active ingredient and propellant. Quality is ensured through testing of components, valves, spray pattern, and other parameters. Dry powder inhalers deliver drug particles without propellants. They must produce respirable aerosol clouds for lung deposition.