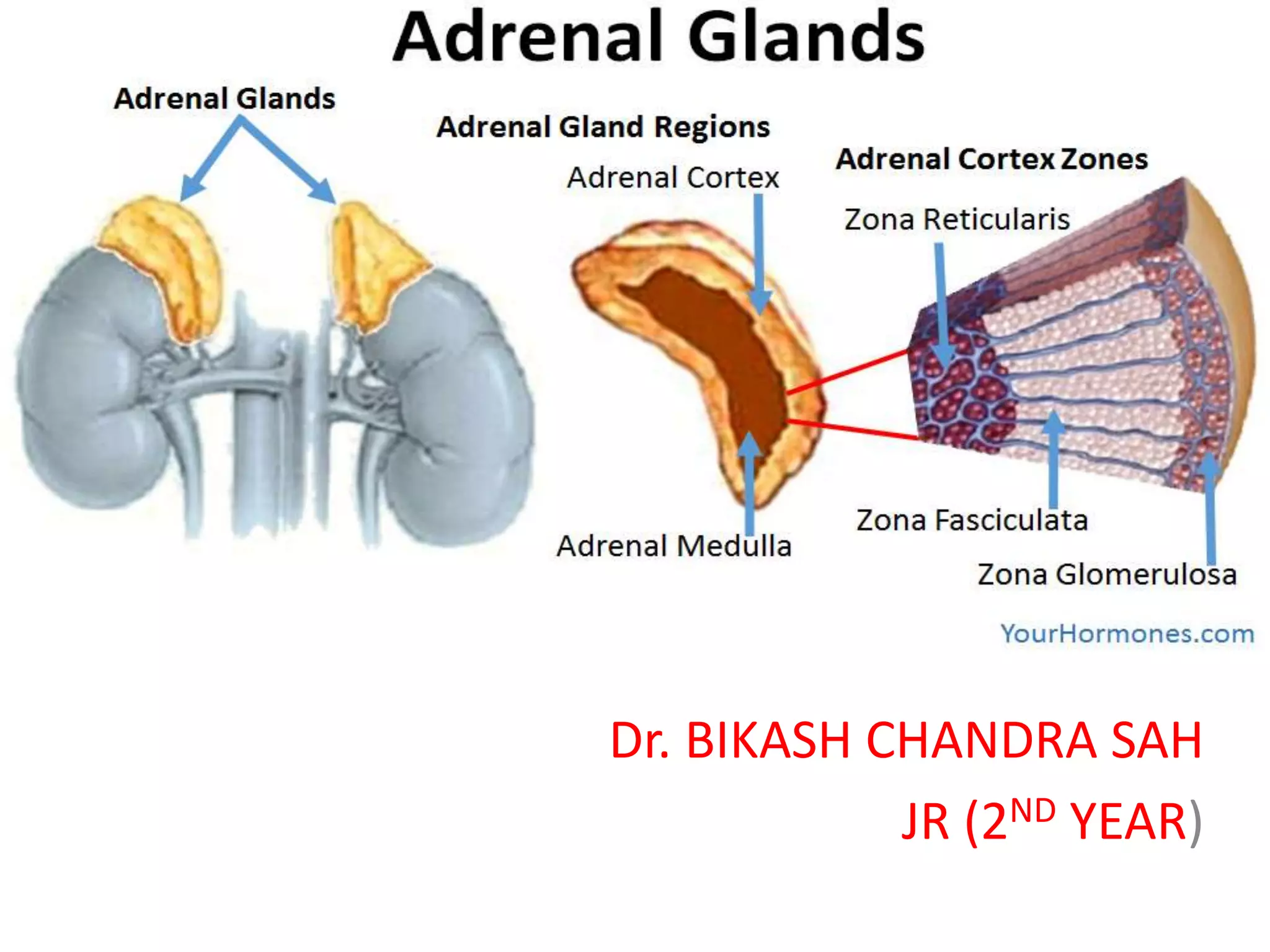
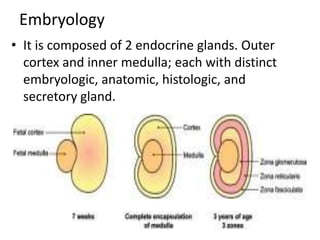
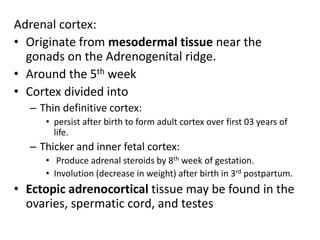
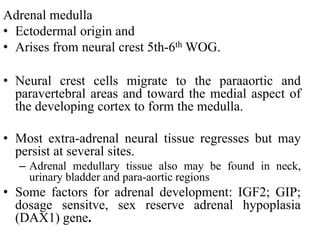
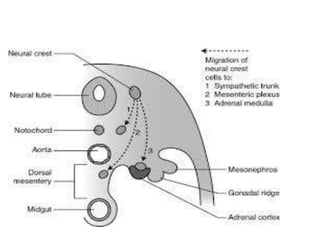
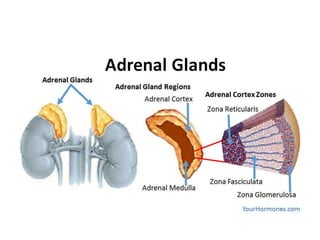
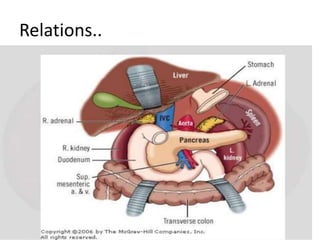
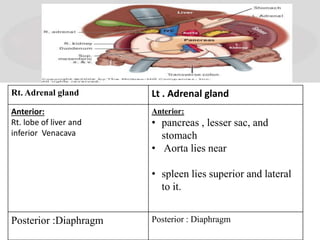
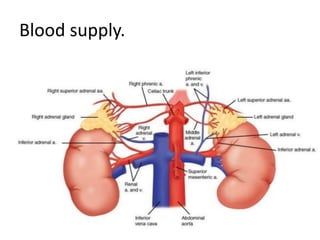
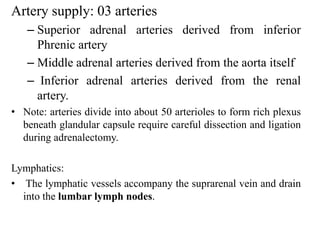
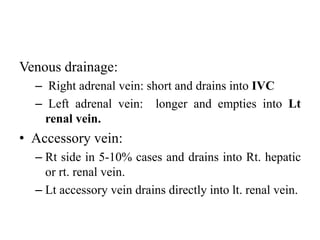
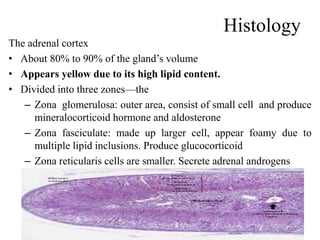
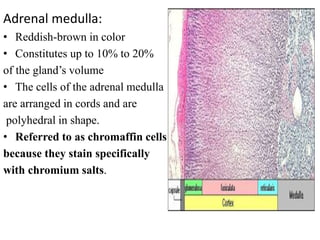
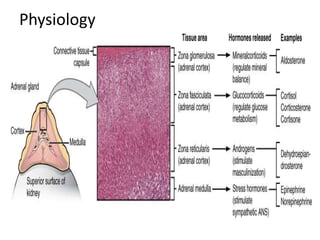
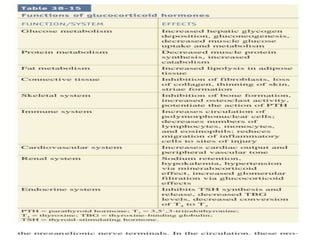
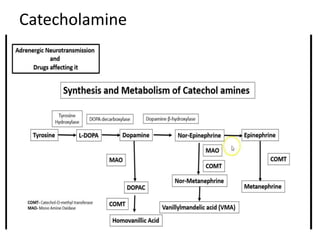
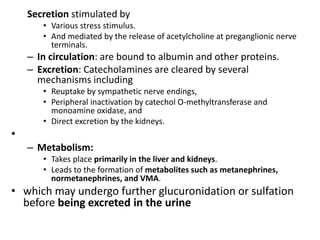
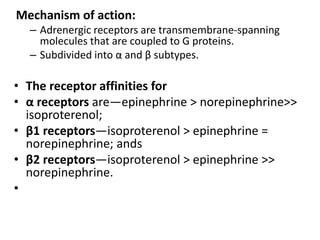
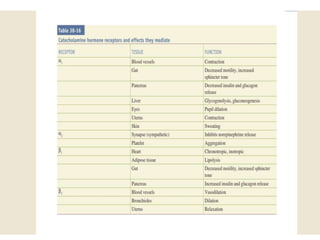
The document provides information on the embryology, anatomy, histology, physiology and functions of the adrenal glands. It discusses that the adrenal cortex and medulla develop from different embryonic origins and produce different hormones. The adrenal cortex consists of three zones that secrete mineralocorticoids, glucocorticoids and sex steroids. The adrenal medulla produces catecholamines. The hormones regulate sodium balance, stress response, growth and sexual development.