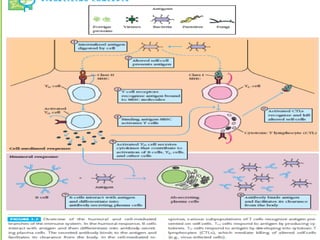
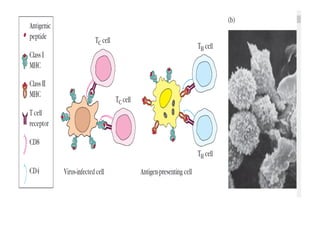
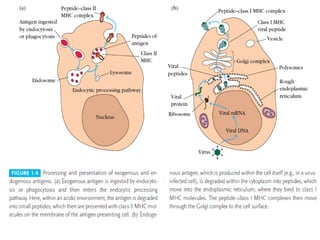
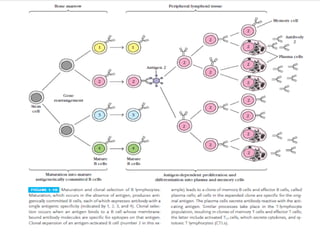
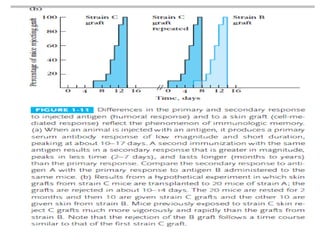
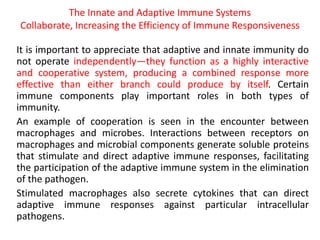
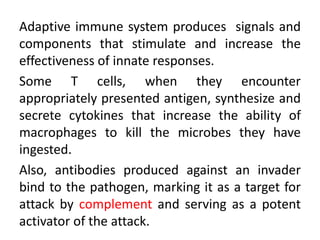
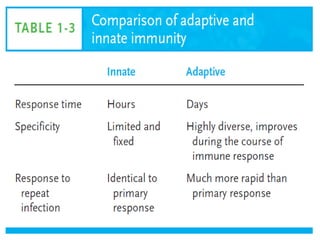
Adaptive immunity can recognize and eliminate specific foreign microorganisms through antigenic specificity, diversity, immunological memory, and self/non-self recognition. It involves lymphocytes (T and B cells) that recognize antigens through cell surface receptors. B cells recognize soluble antigens and produce antibodies, while T cells recognize antigen peptides bound to MHC molecules on cell surfaces and carry out cell-mediated immunity. Antigens are processed within cells and presented on MHC molecules to activate lymphocytes in an antigen-specific manner, causing clonal expansion of lymphocytes that recognize that antigen.