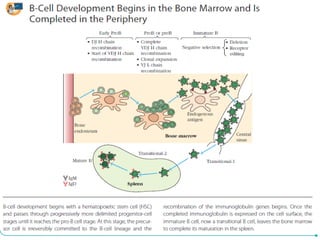
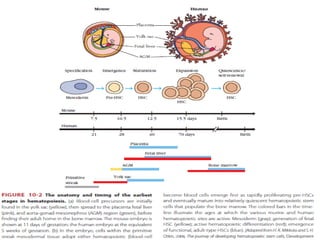
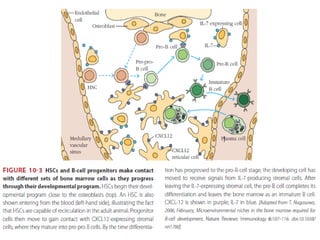
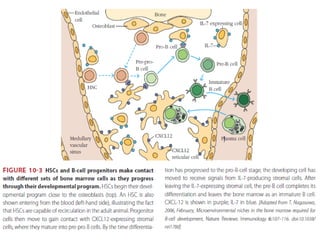
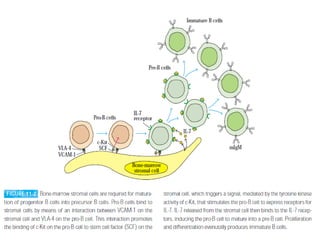
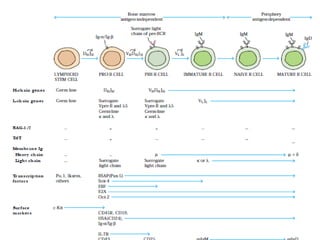
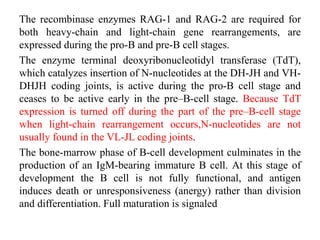
B-cell development begins with common lymphoid progenitors in the bone marrow that can develop into either B or T cells. Most progenitors become B cells and undergo differentiation by expressing cell surface molecules in response to signals. Developing B cells migrate through stages to become immature B cells that leave the marrow. Mature B cells secrete antibodies and their development involves rearrangement of immunoglobulin genes. B-cell development occurs differently in the fetal liver versus adult bone marrow.