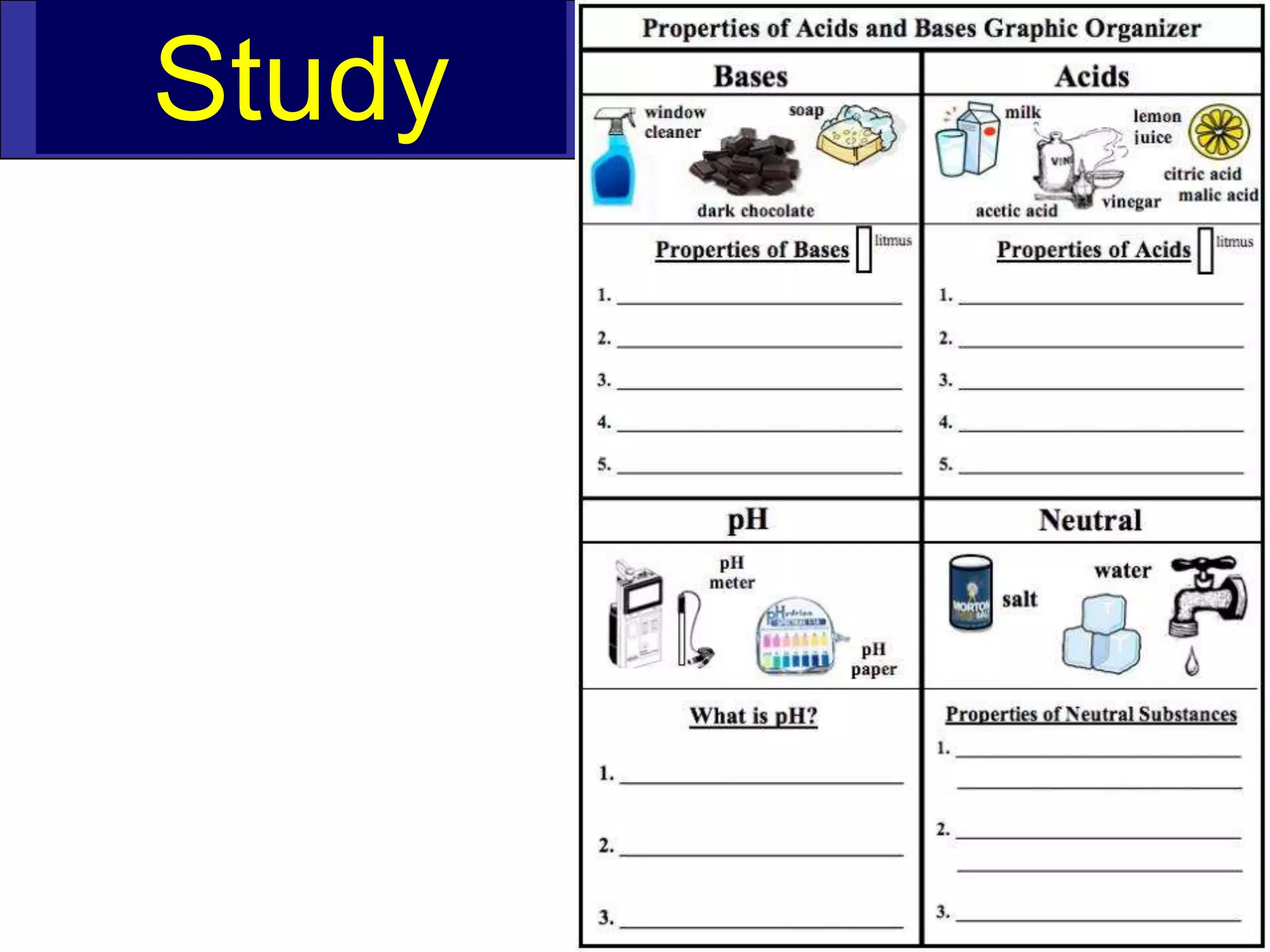
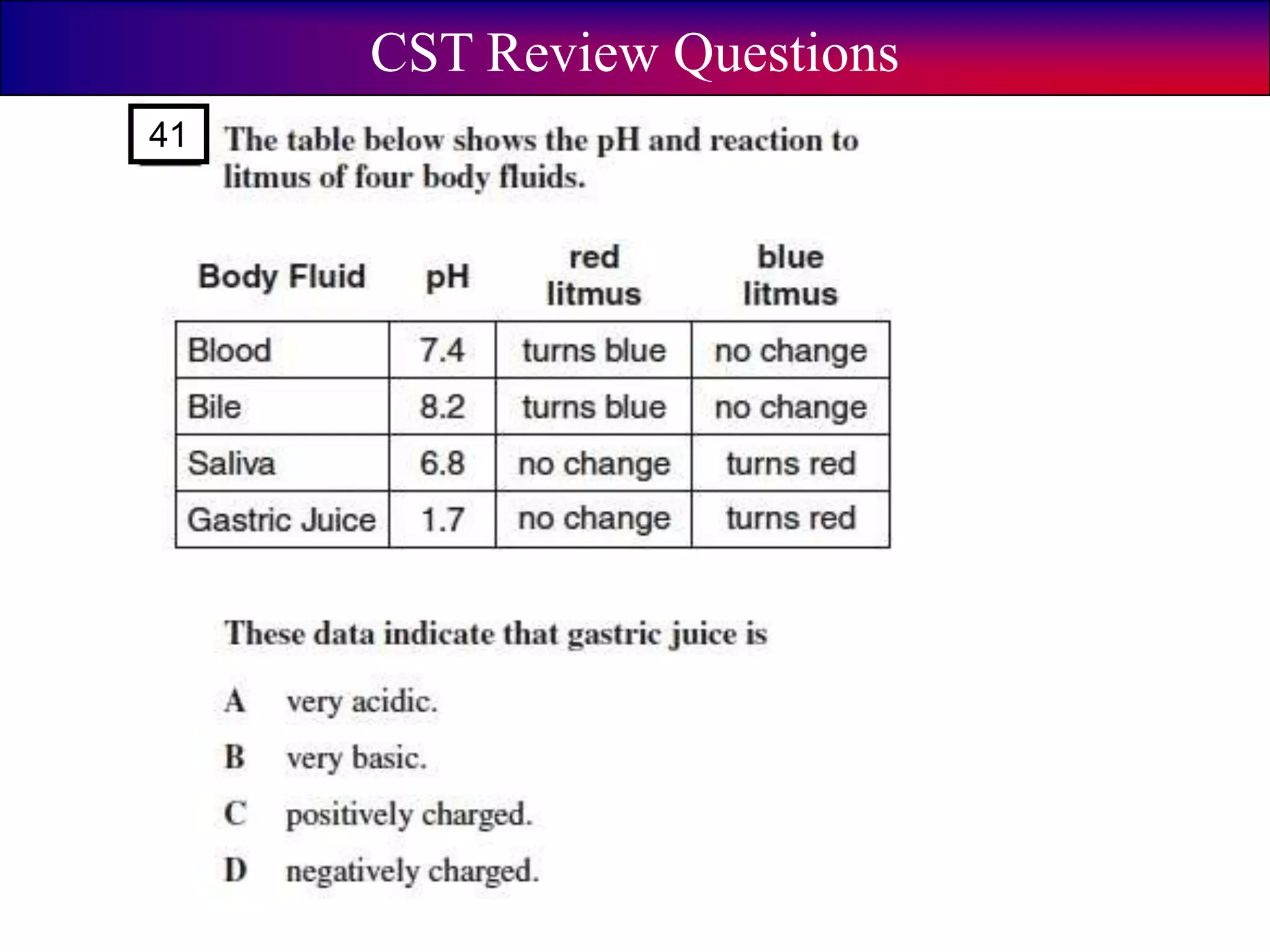
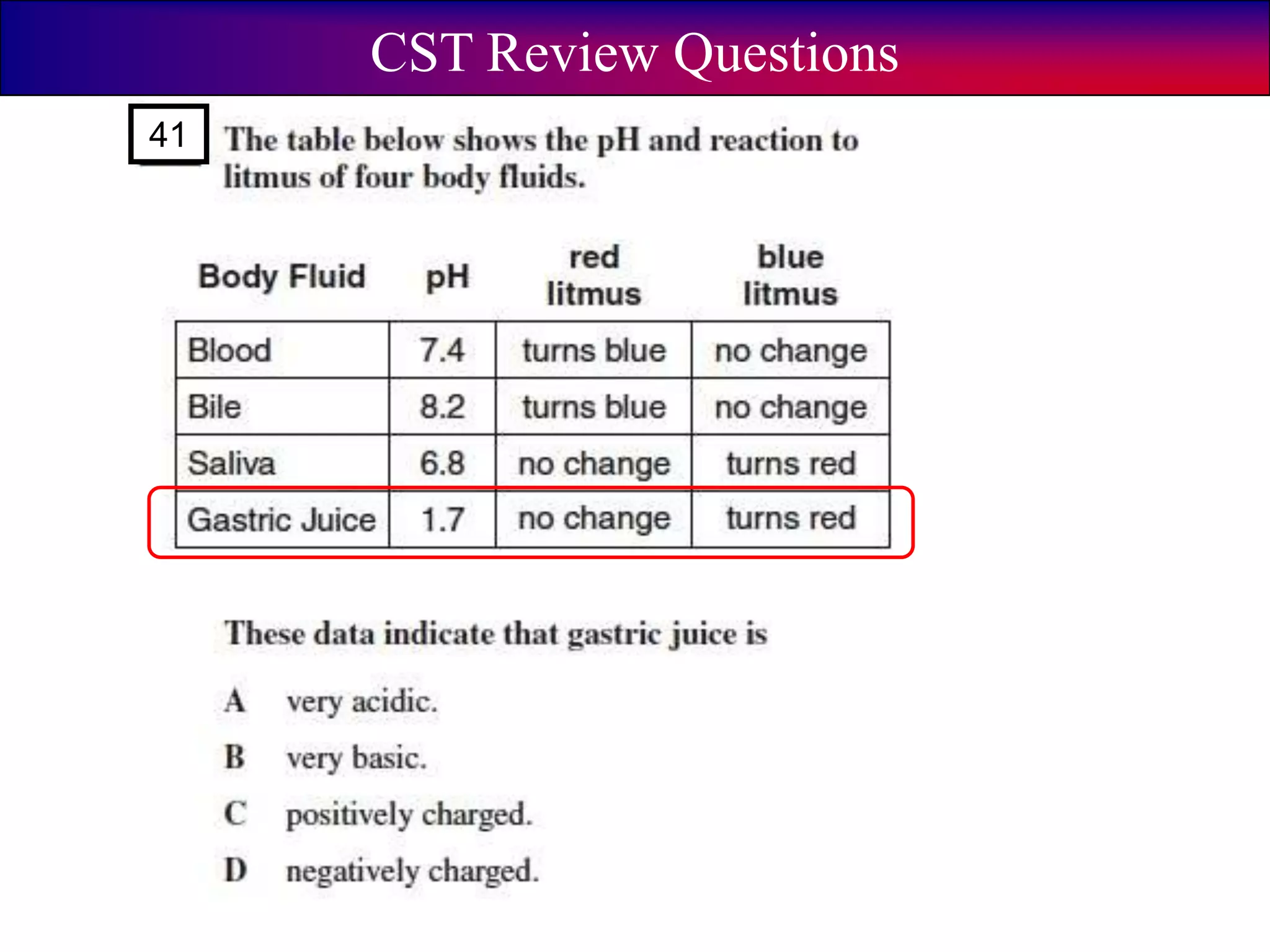
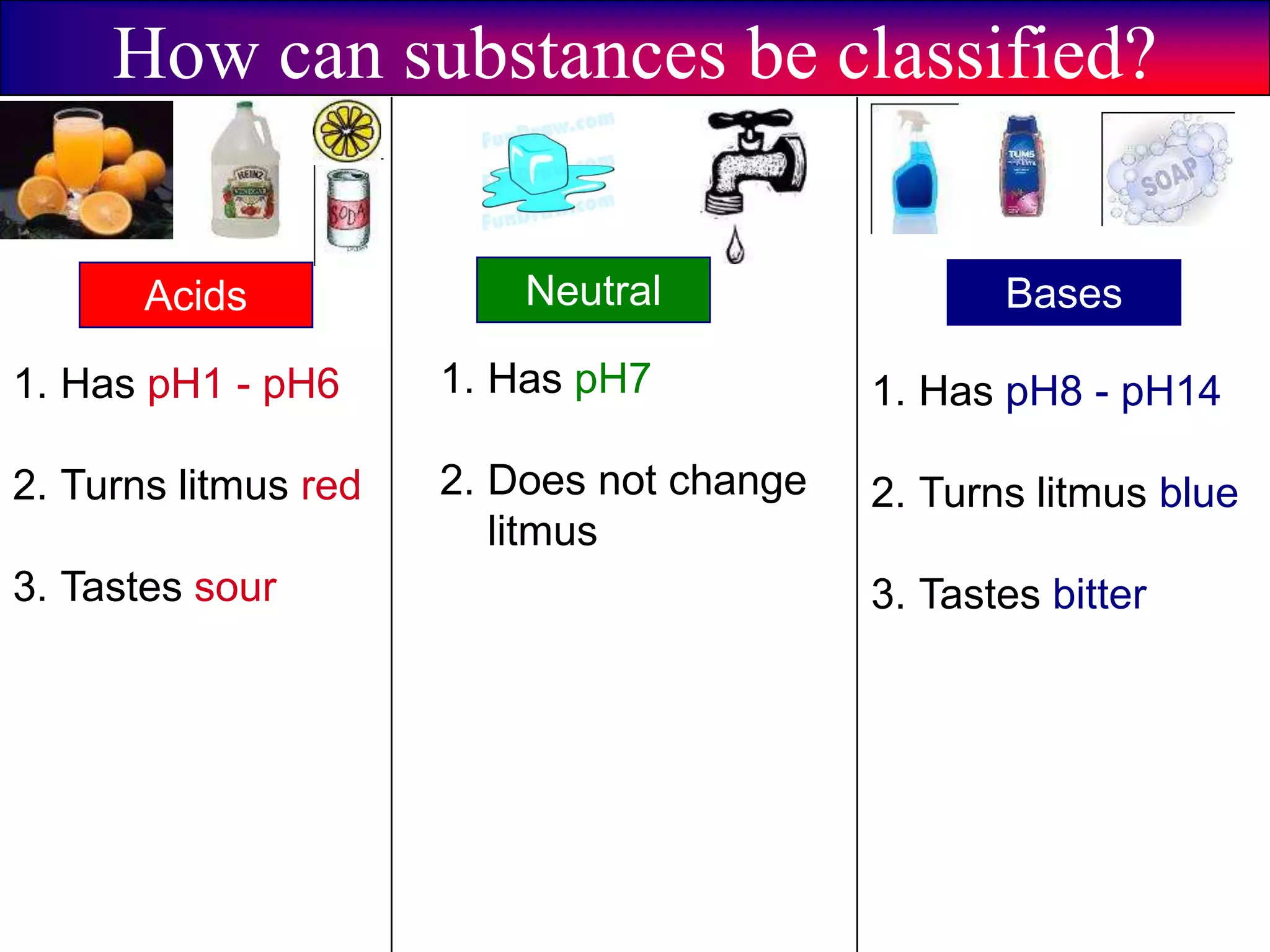
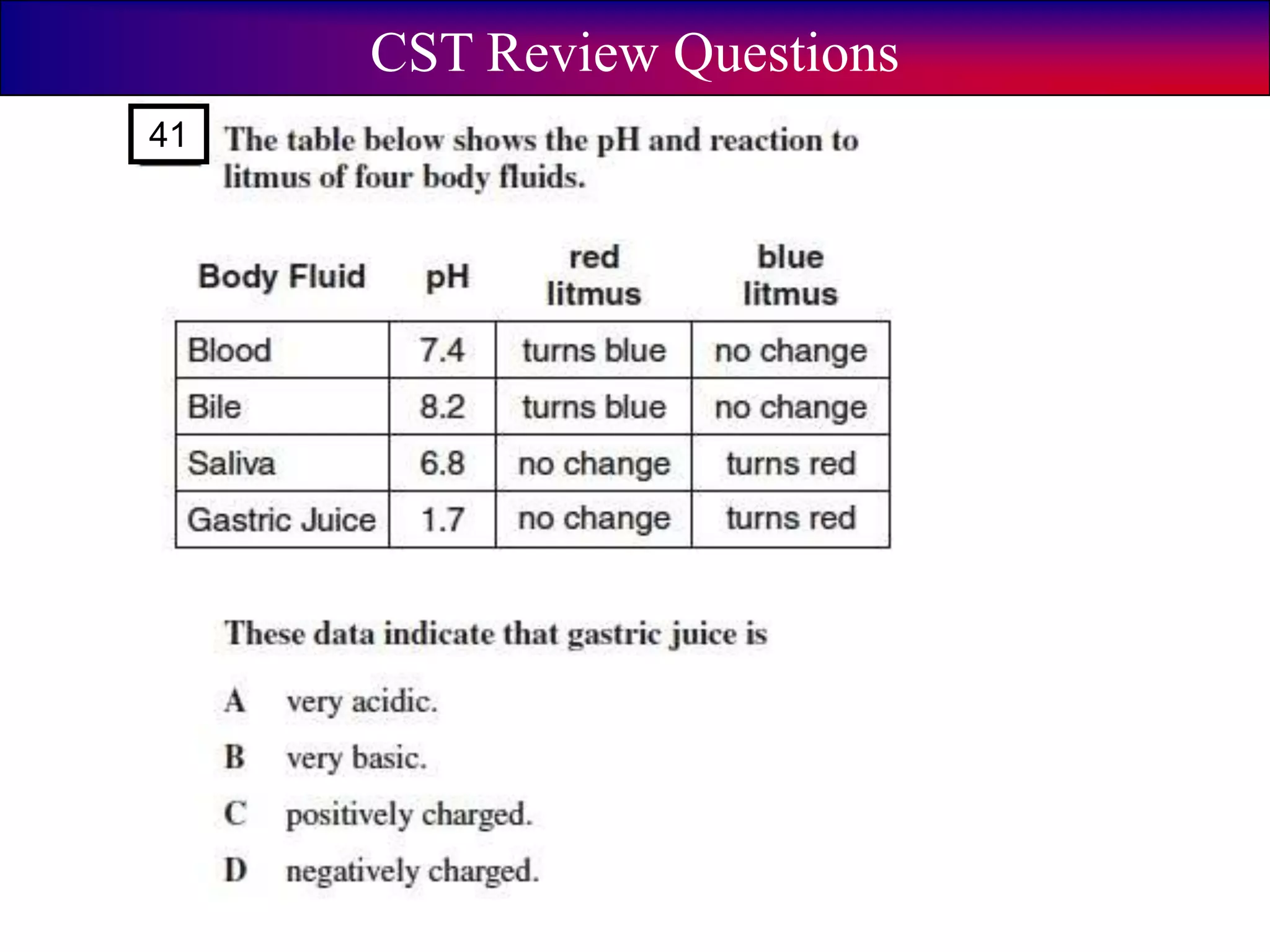
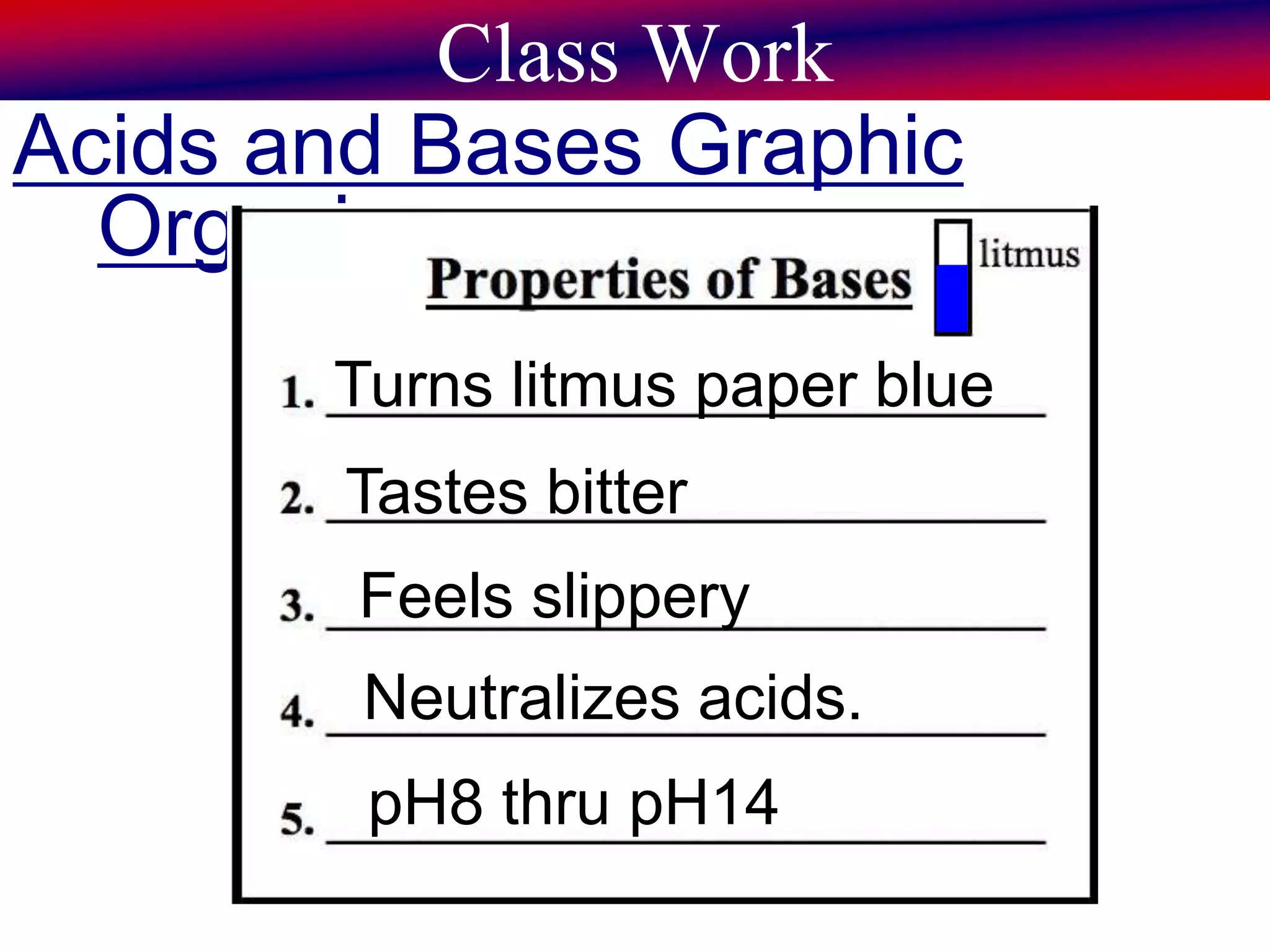
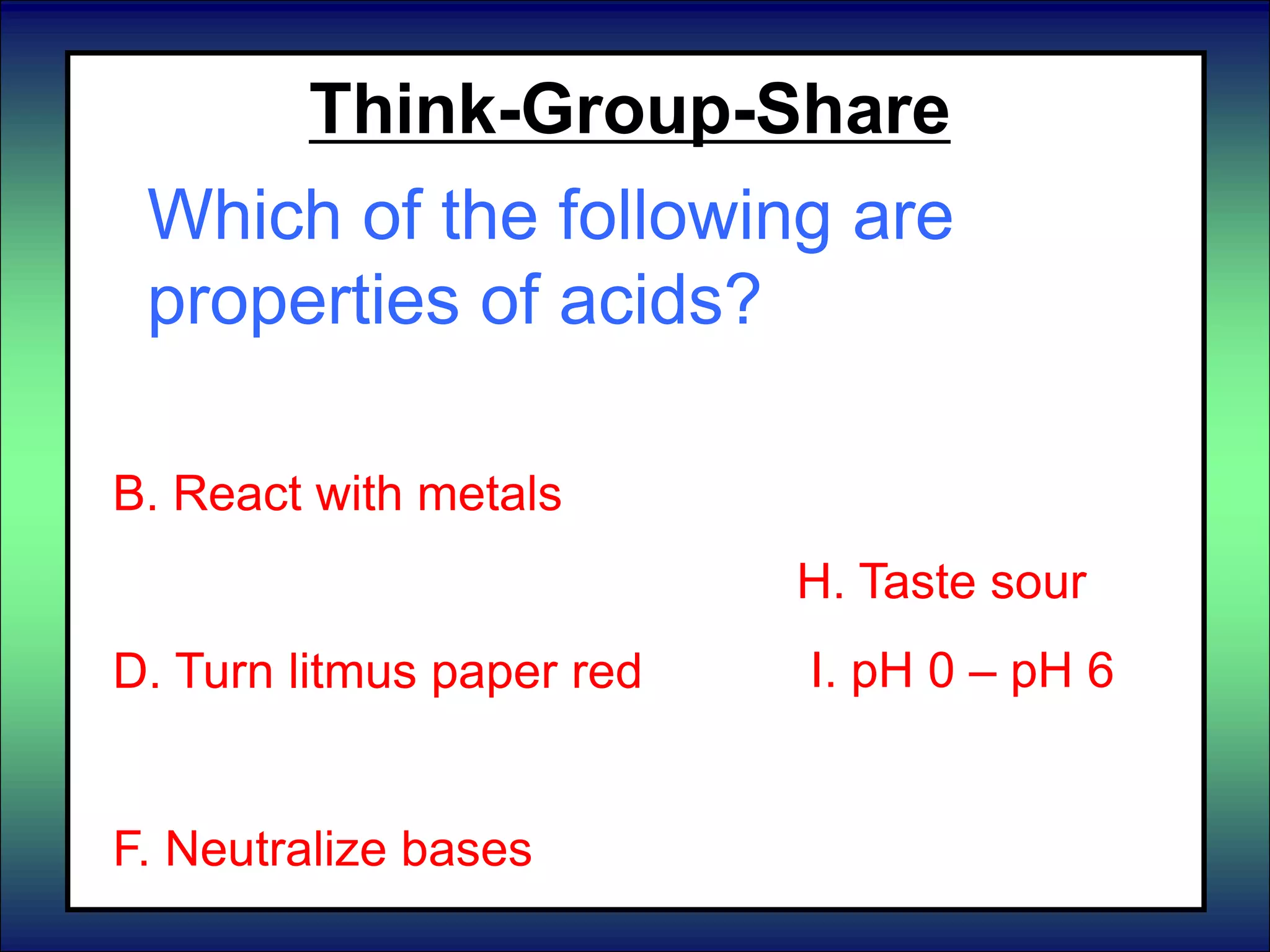
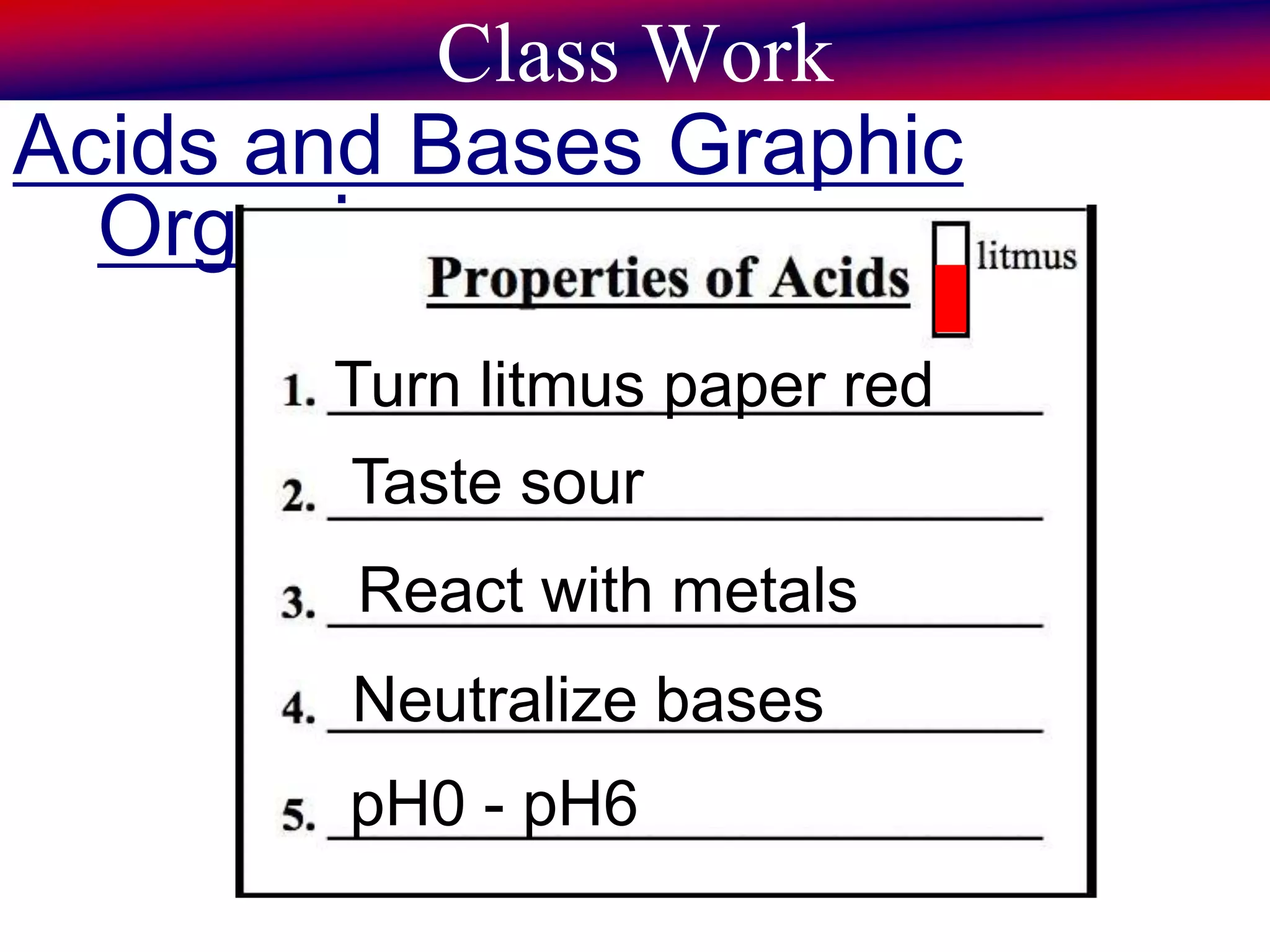
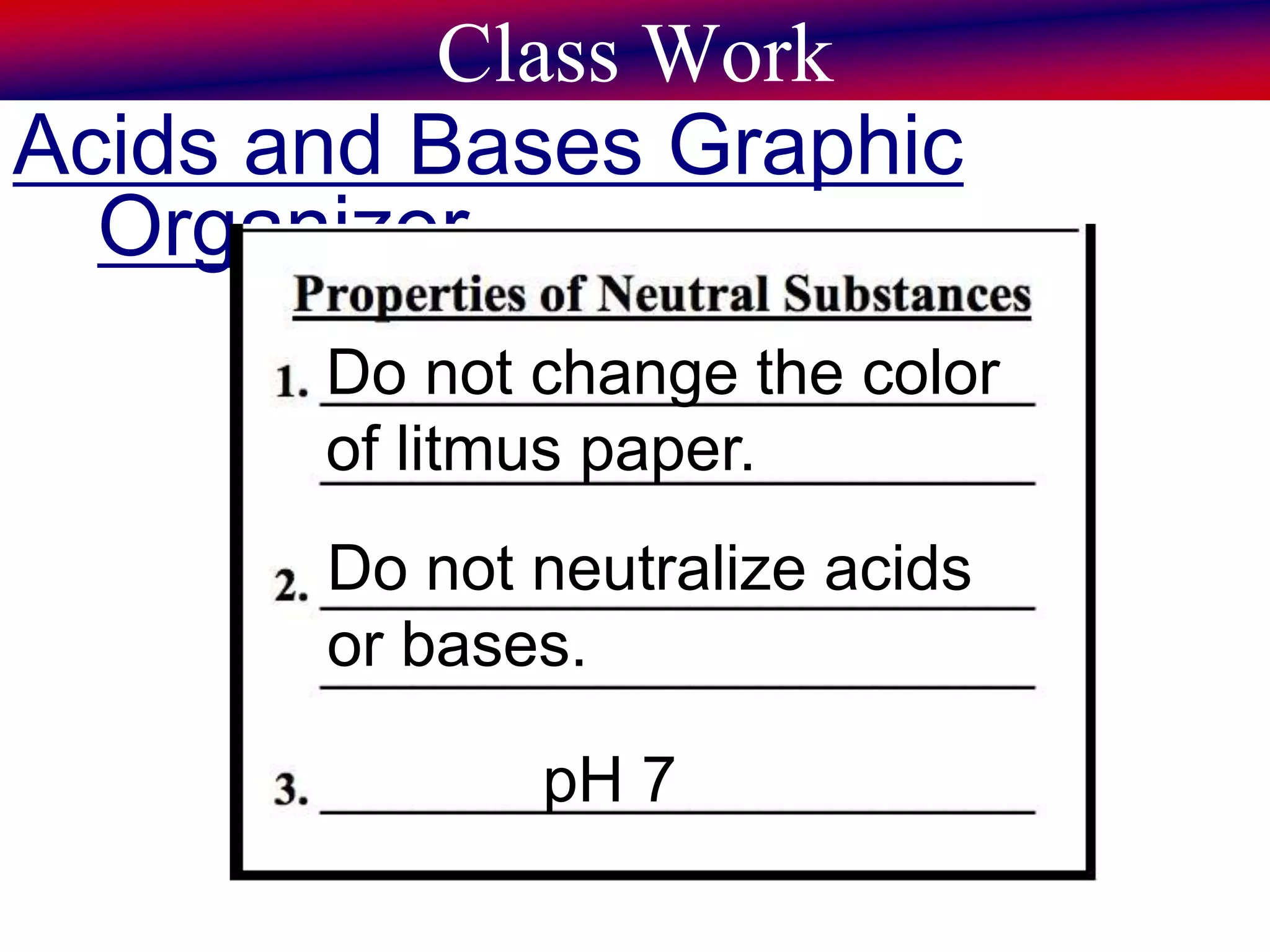
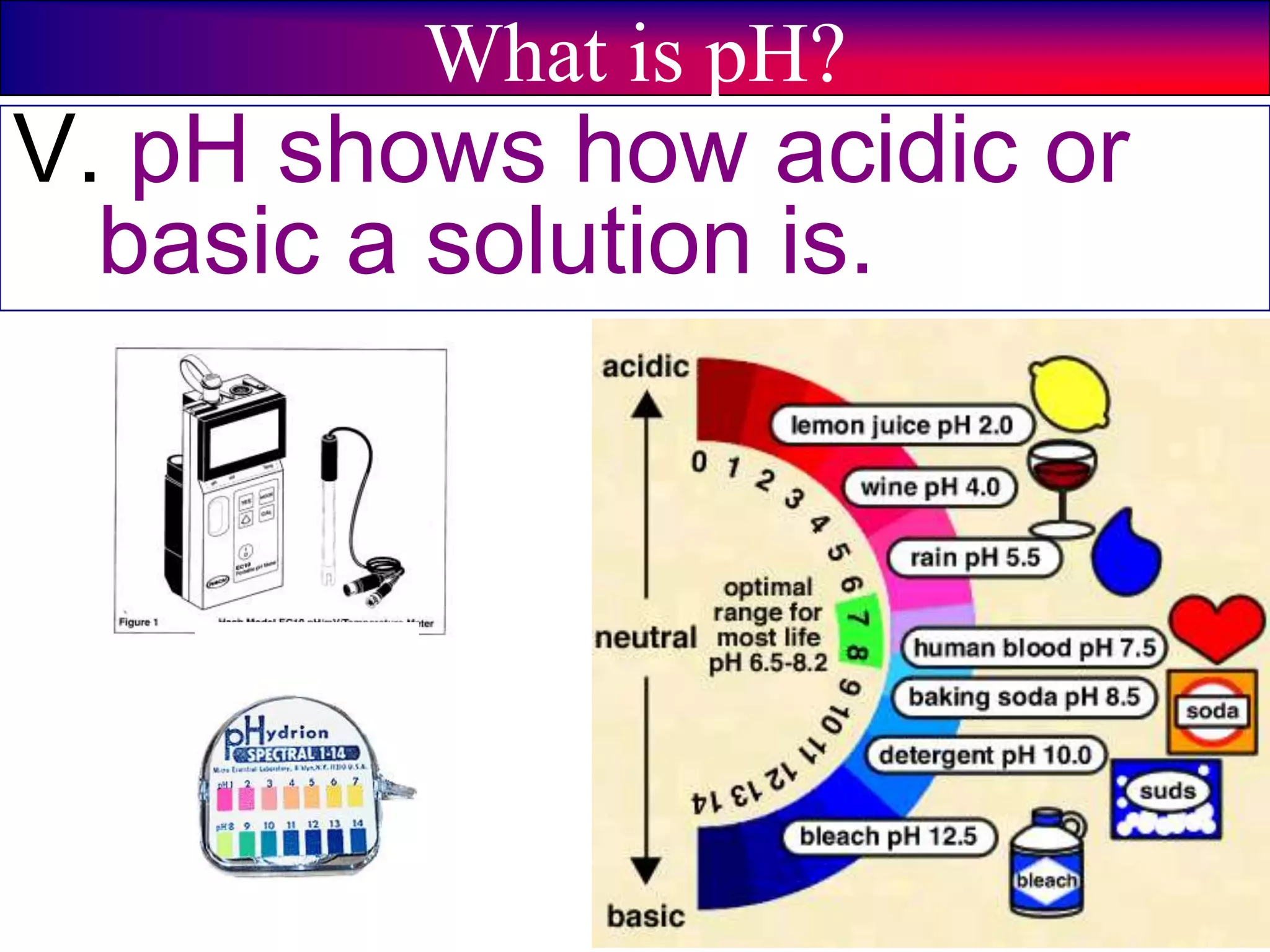
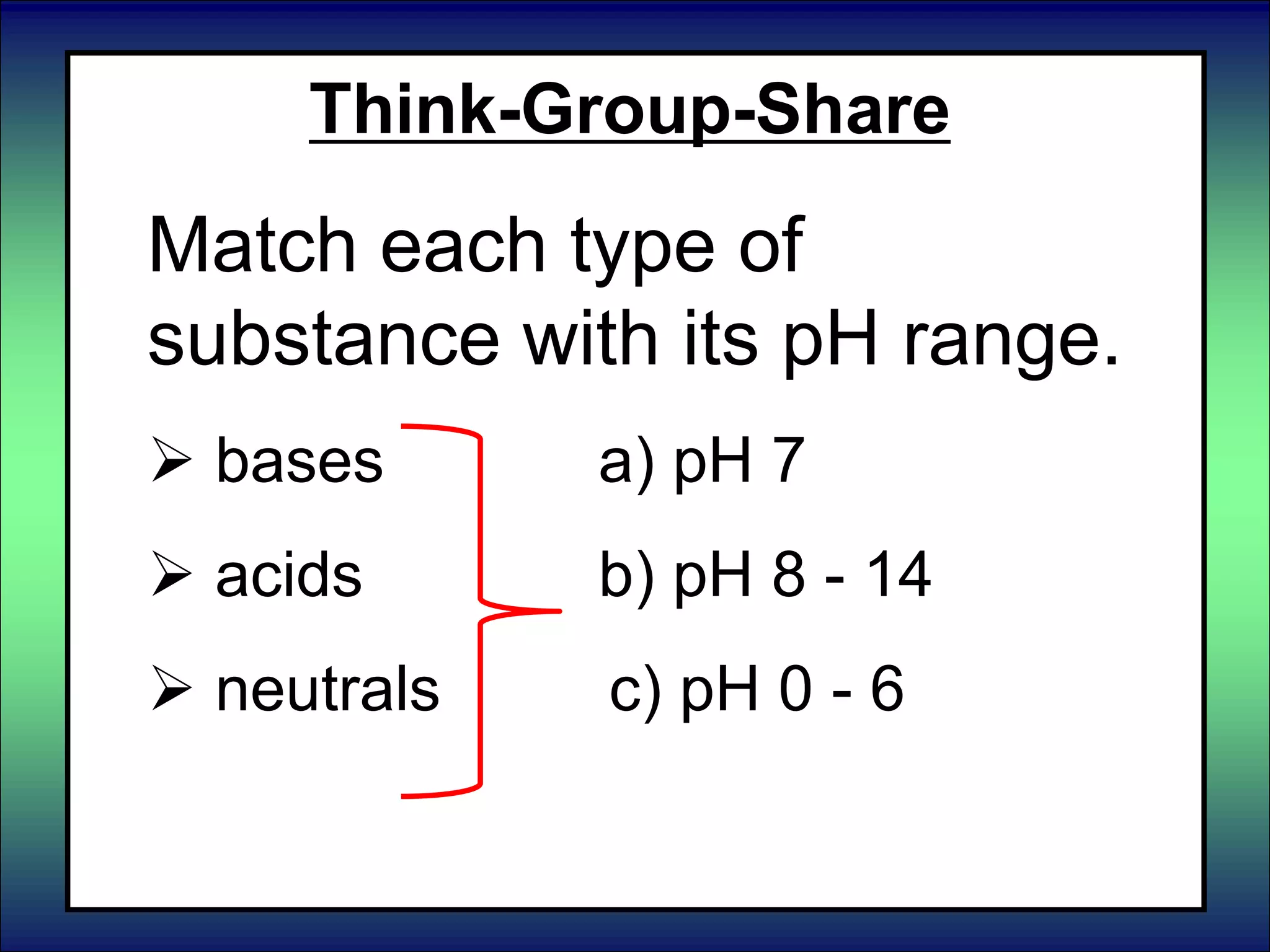
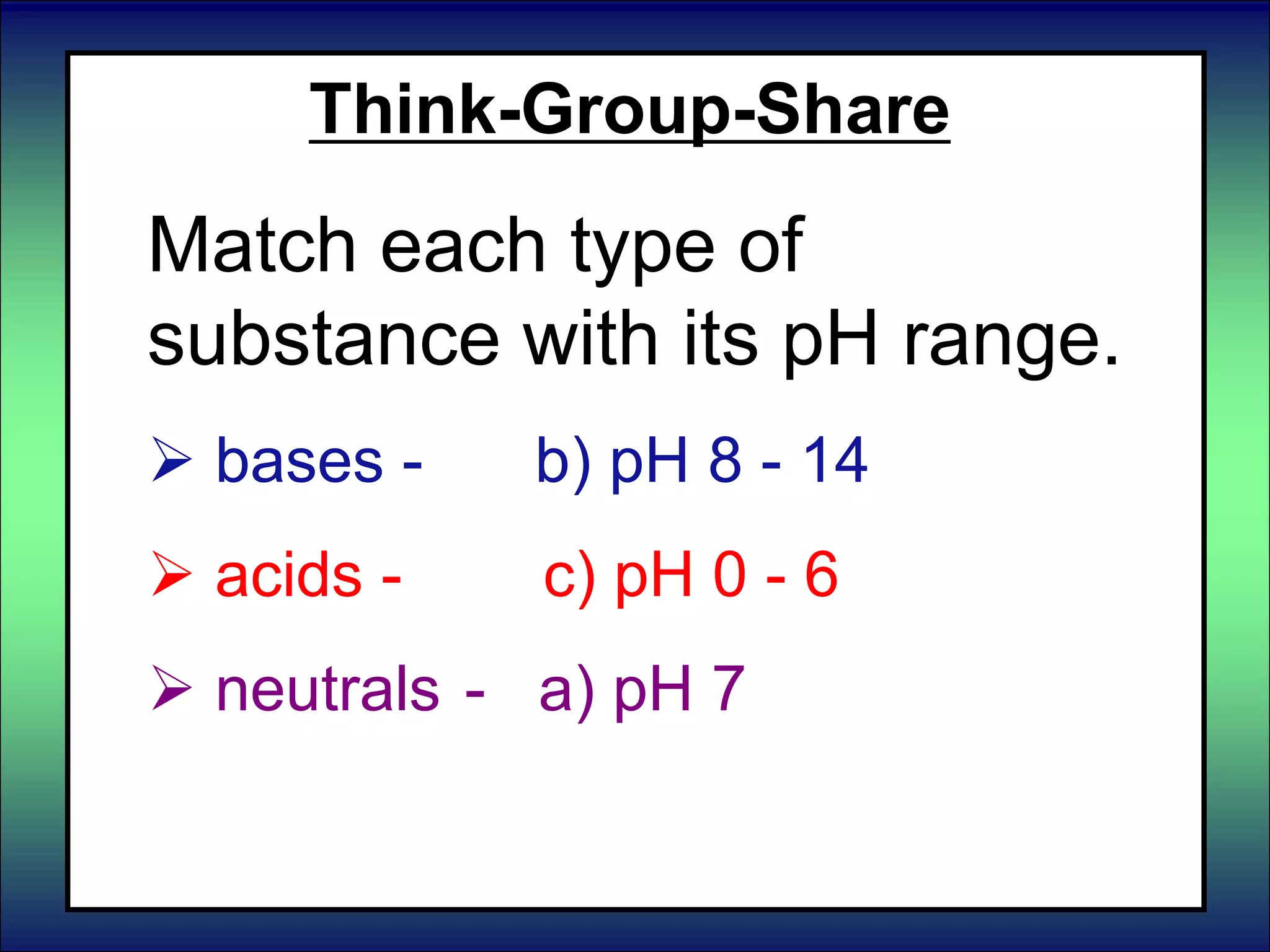
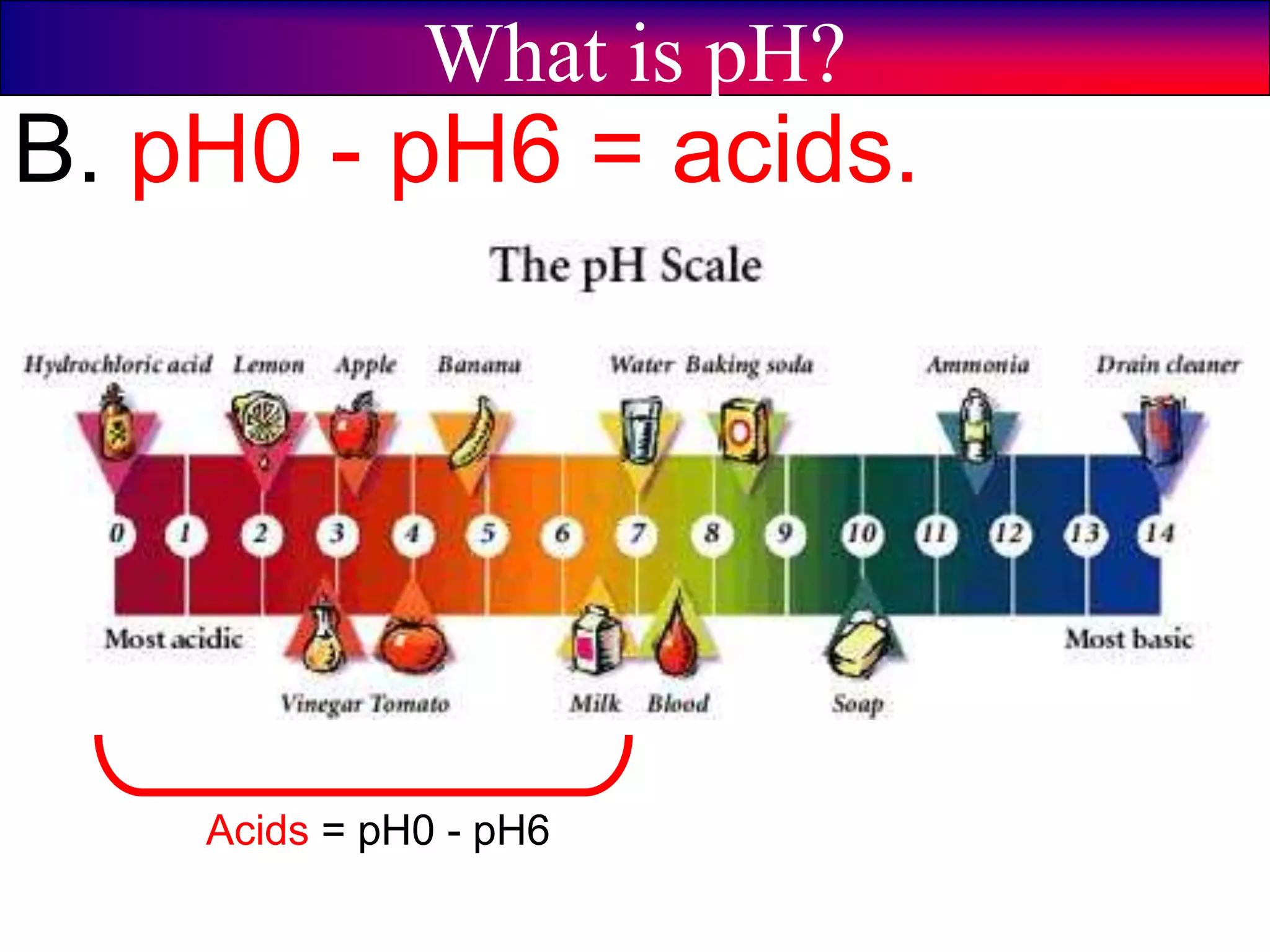
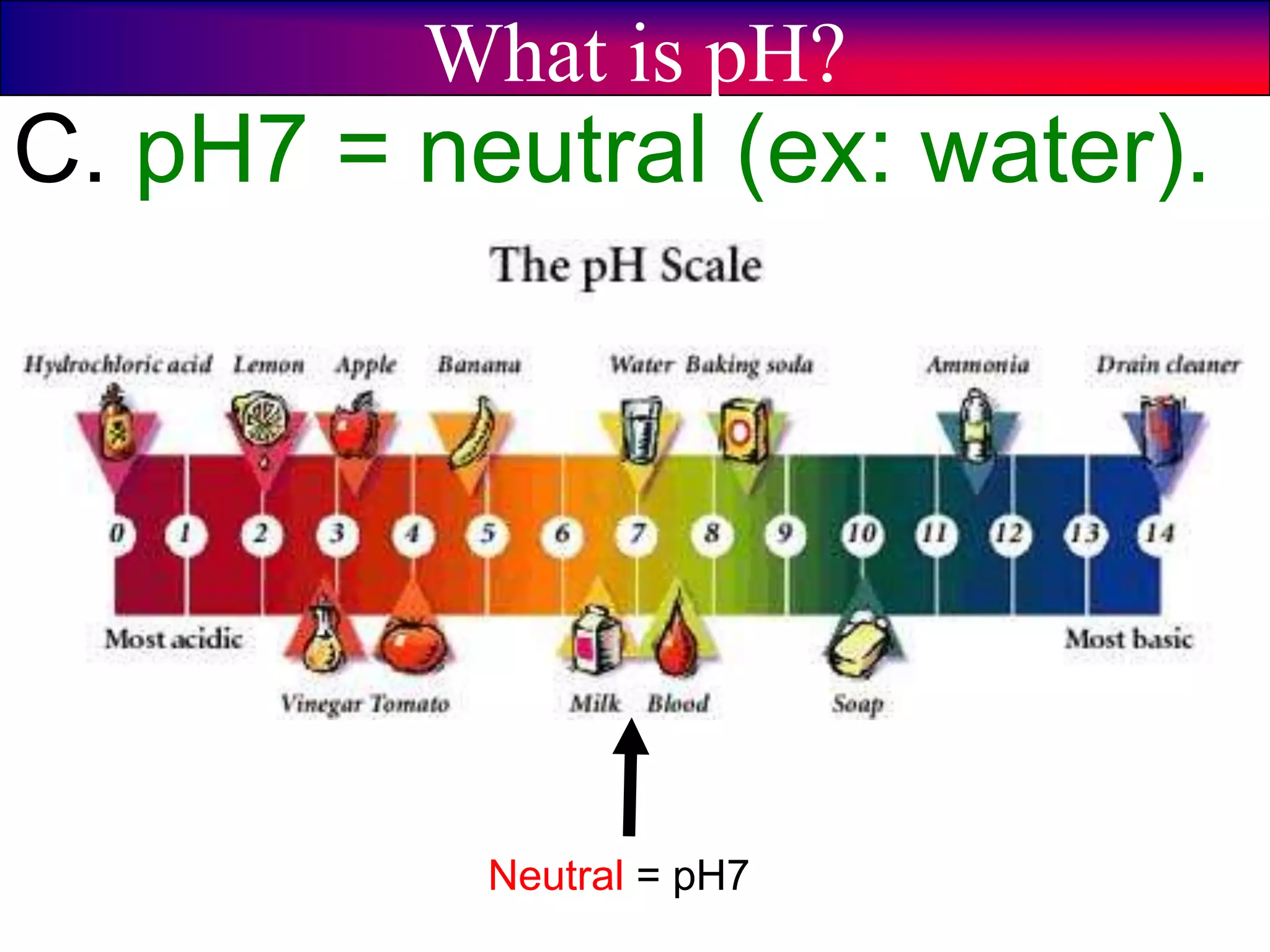
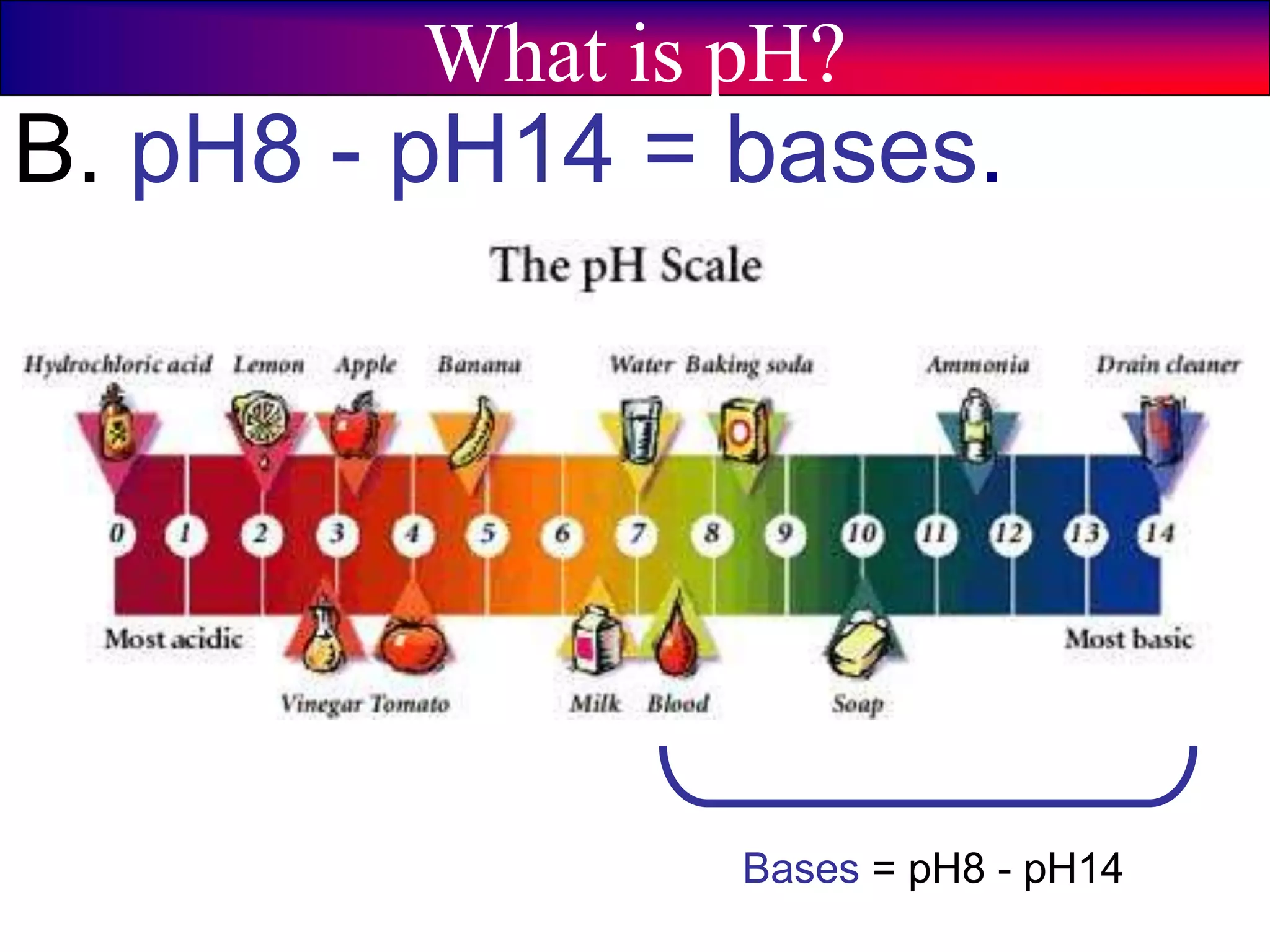
This document discusses acids, bases, and pH. It begins by defining the three classifications of substances: acids, bases, and neutrals. Acids have a pH between 0-6, turn litmus paper red, and taste sour. Bases have a pH between 8-14, turn litmus paper blue, and taste bitter. Neutrals have a pH of 7 and do not change the color of litmus paper. It then discusses properties of each classification and how pH is used to indicate how acidic or basic a substance is.