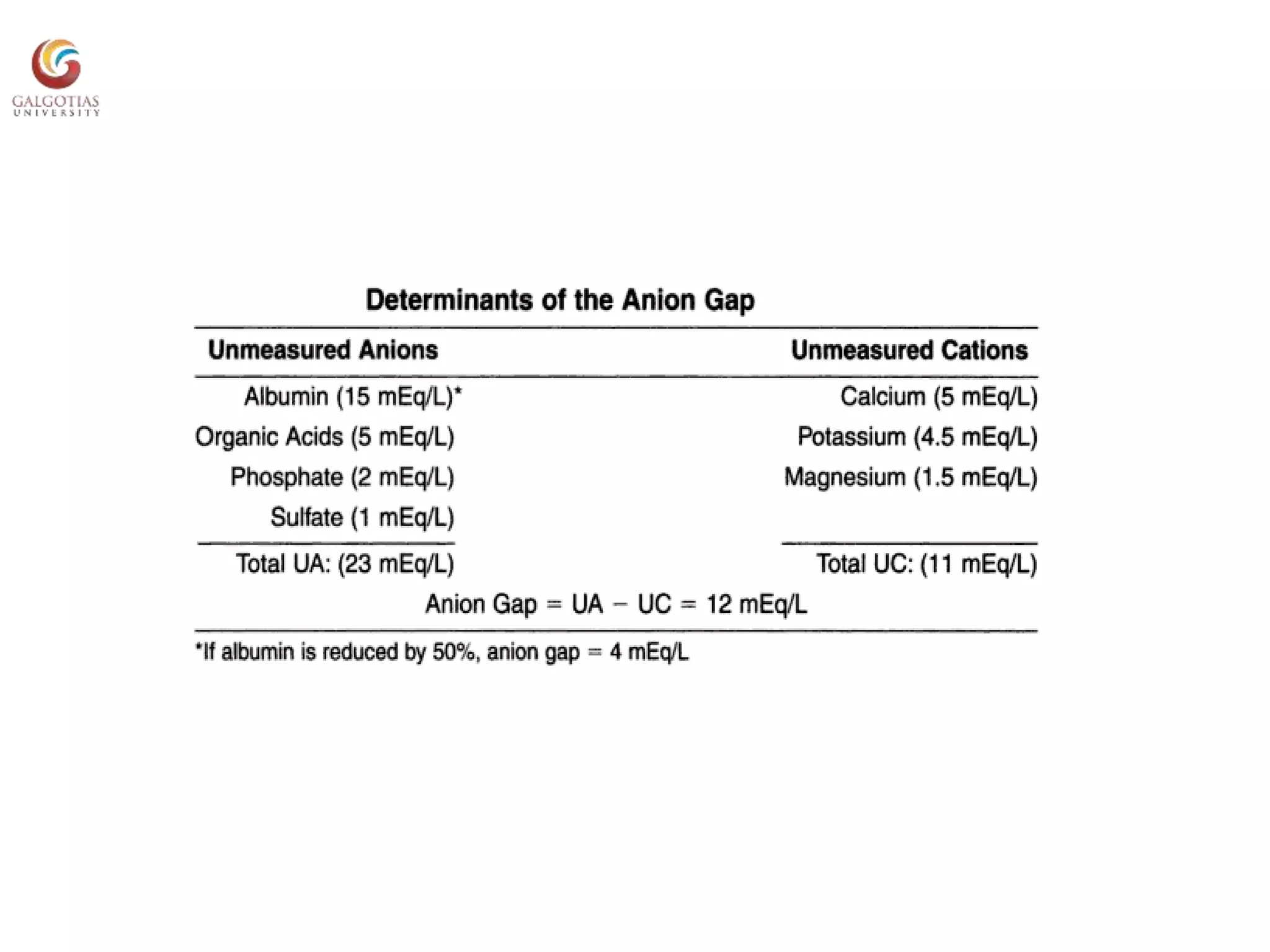
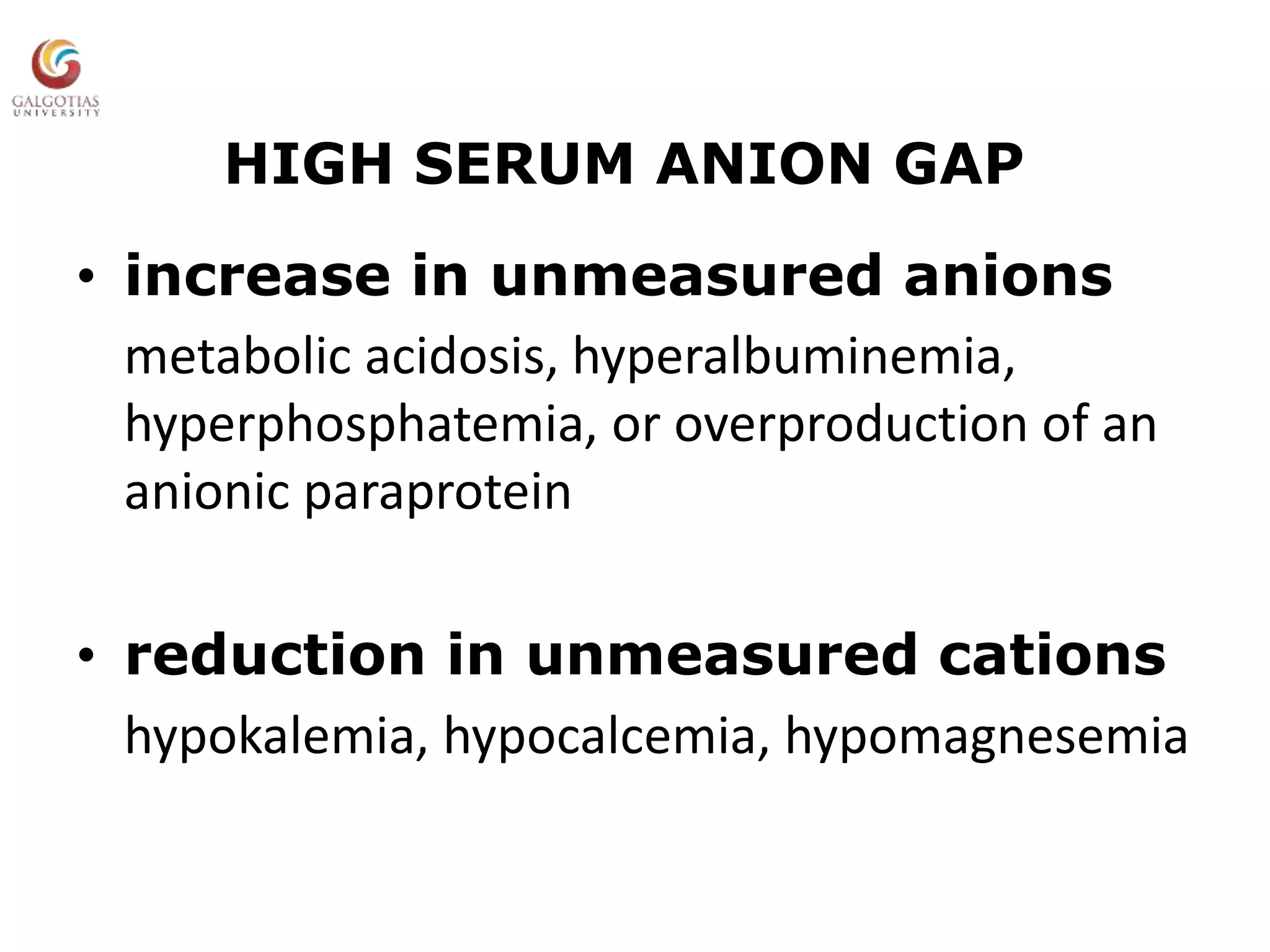
This document provides an overview of acid-base balance, including normal pH levels and buffer systems that regulate pH. It defines different acid-base disorders like metabolic acidosis, metabolic alkalosis, respiratory acidosis, and respiratory alkalosis. For each disorder it discusses compensatory responses and common causes. Key concepts covered are the Henderson-Hasselbach equation, anion gap, factors that can cause a rise or fall in bicarbonate levels, and how the kidneys and lungs work to compensate for acid-base imbalances. Reference materials are also listed.


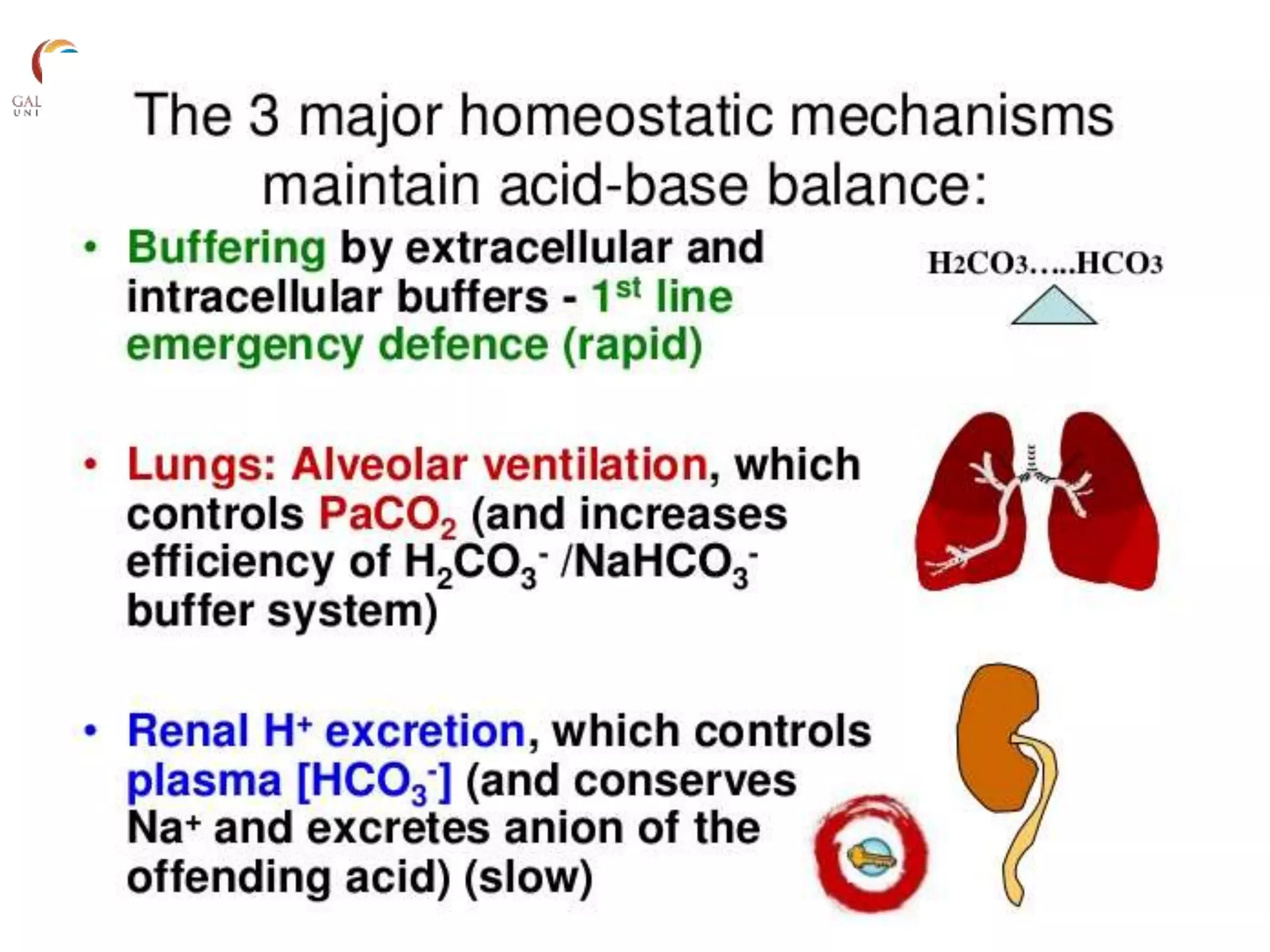


![Henderson-Hasselbach Equation
• pH=6.1+log
pH 7.00 7.40 7.70
[H+]
nmol/L 100 40 20
[HCO3- ] (mEq/L)
0.03XpCO2 (mm Hg)](https://image.slidesharecdn.com/acidbasebalance-190921163659/75/Acid-base-balance-8-2048.jpg)




![Serum Anion Gap
[Na+] - ([Cl-]+[HCO3
- ])
9 ± 3 mEq/L (mmol/L)](https://image.slidesharecdn.com/acidbasebalance-190921163659/75/Acid-base-balance-16-2048.jpg)