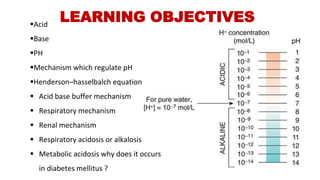
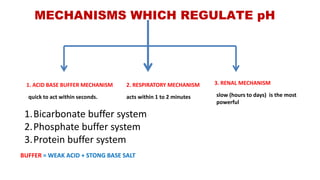
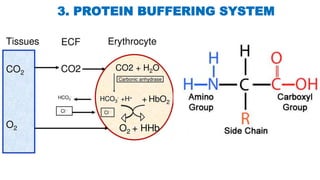
The document discusses the concept of pH and buffer systems in the body, detailing the roles of acids and bases, regulation mechanisms, and implications of pH imbalances such as acidosis and alkalosis. It describes the Henderson-Hasselbalch equation, various buffer systems, and the roles of the respiratory and renal systems in maintaining pH homeostasis, particularly in conditions like diabetes mellitus. Additionally, it identifies causes and physiological impacts of metabolic acidosis and alkalosis.