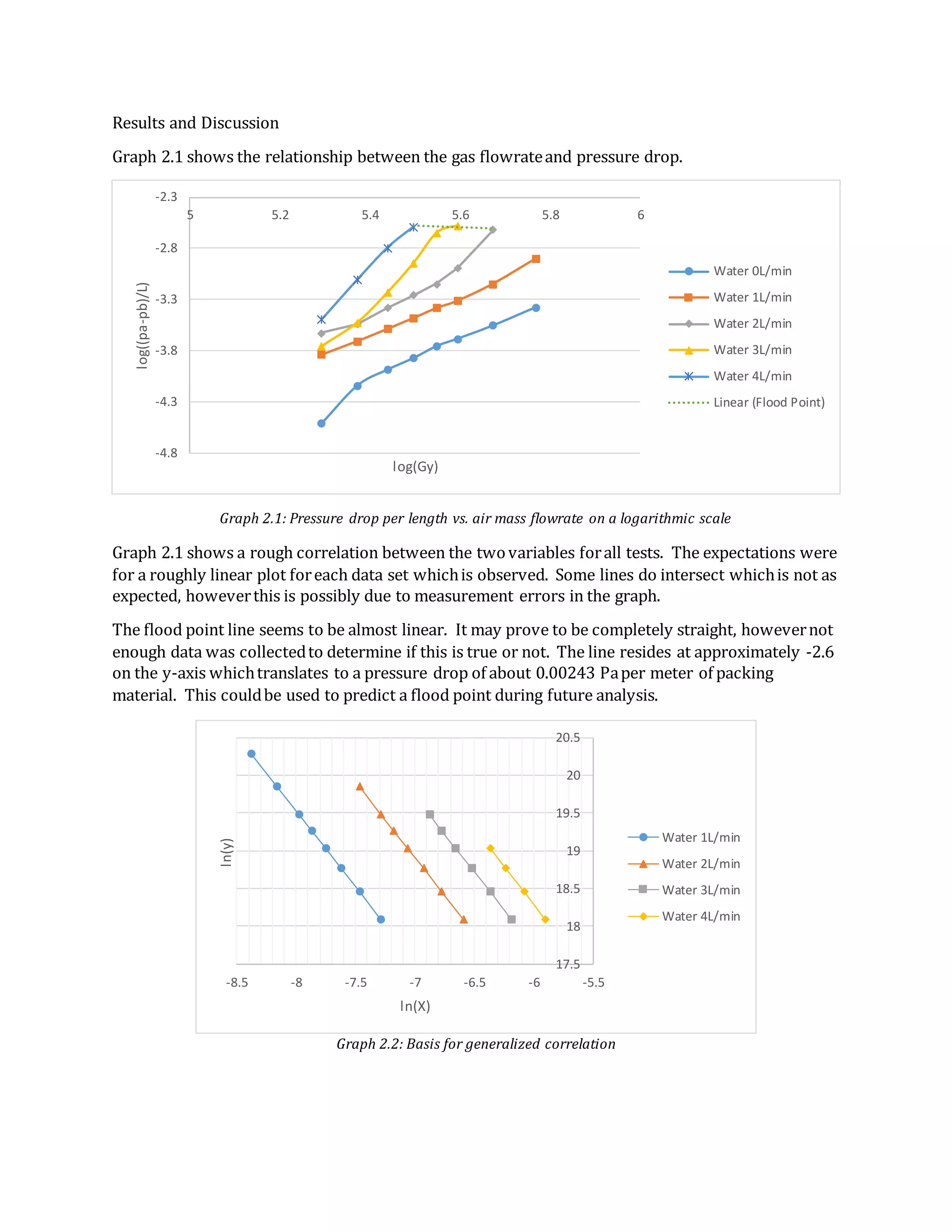
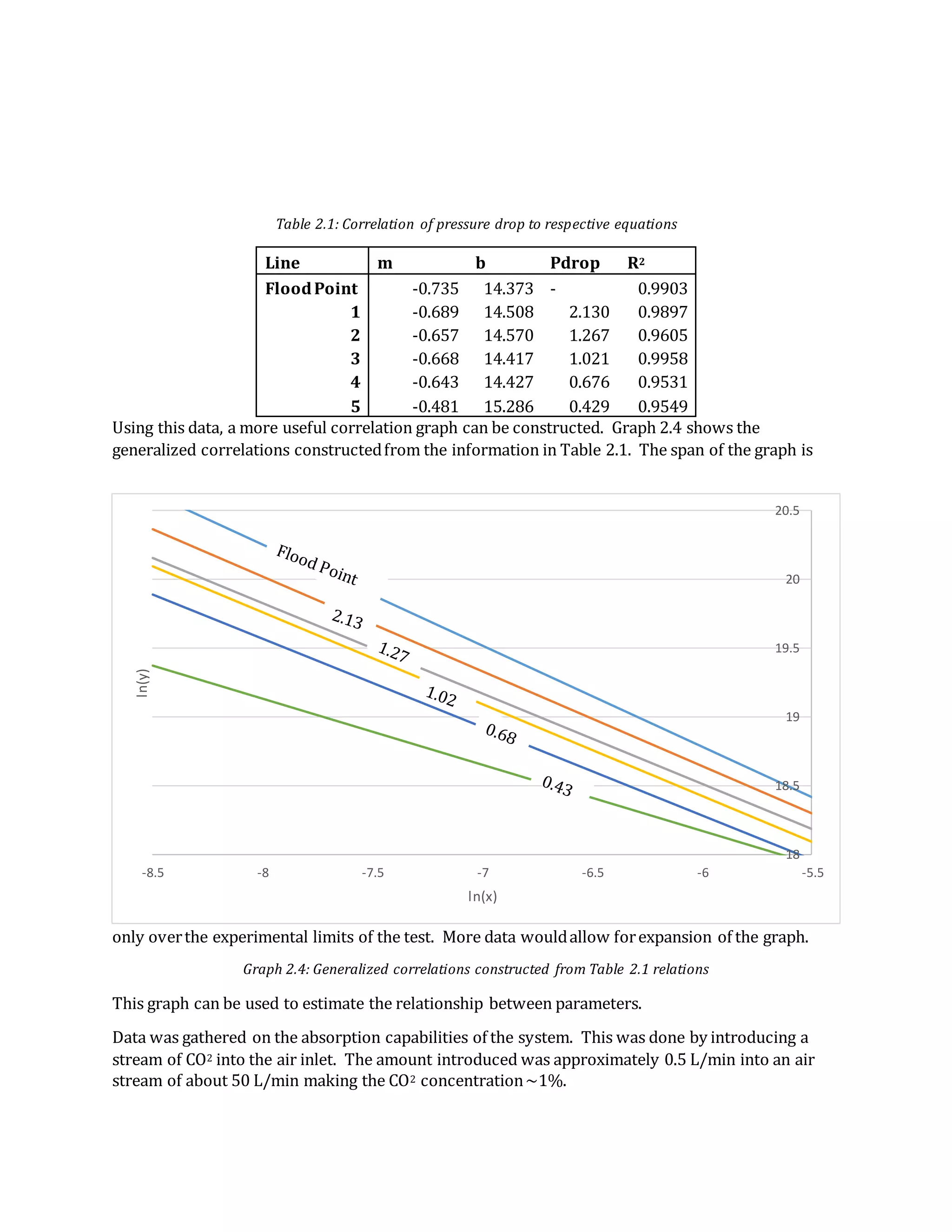
- The document summarizes an experiment on absorption processes using a countercurrent gas-liquid absorption tower.
- Key findings include developing relationships between liquid flow rate, vapor flow rate, and pressure drop. Generalized correlations were constructed from these relationships.
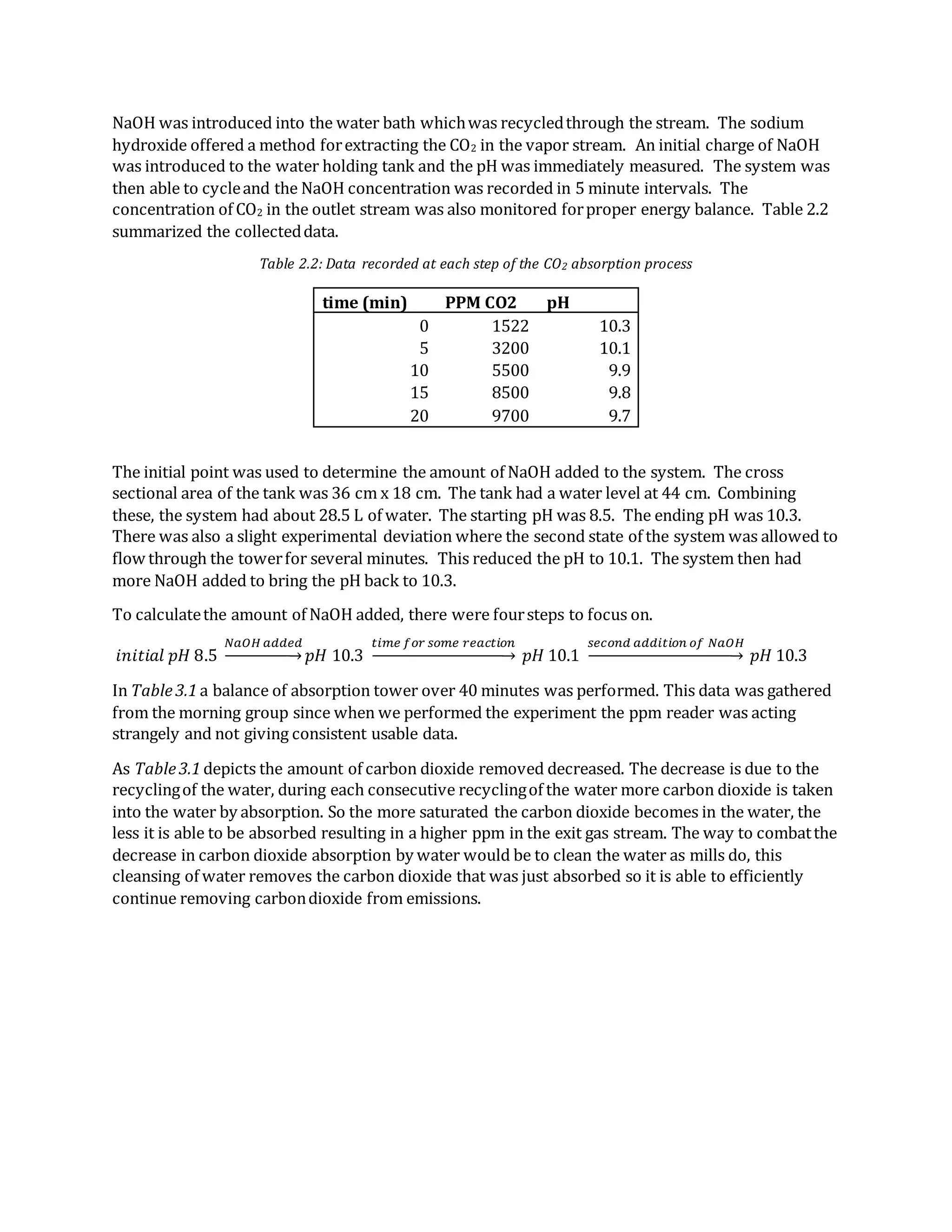
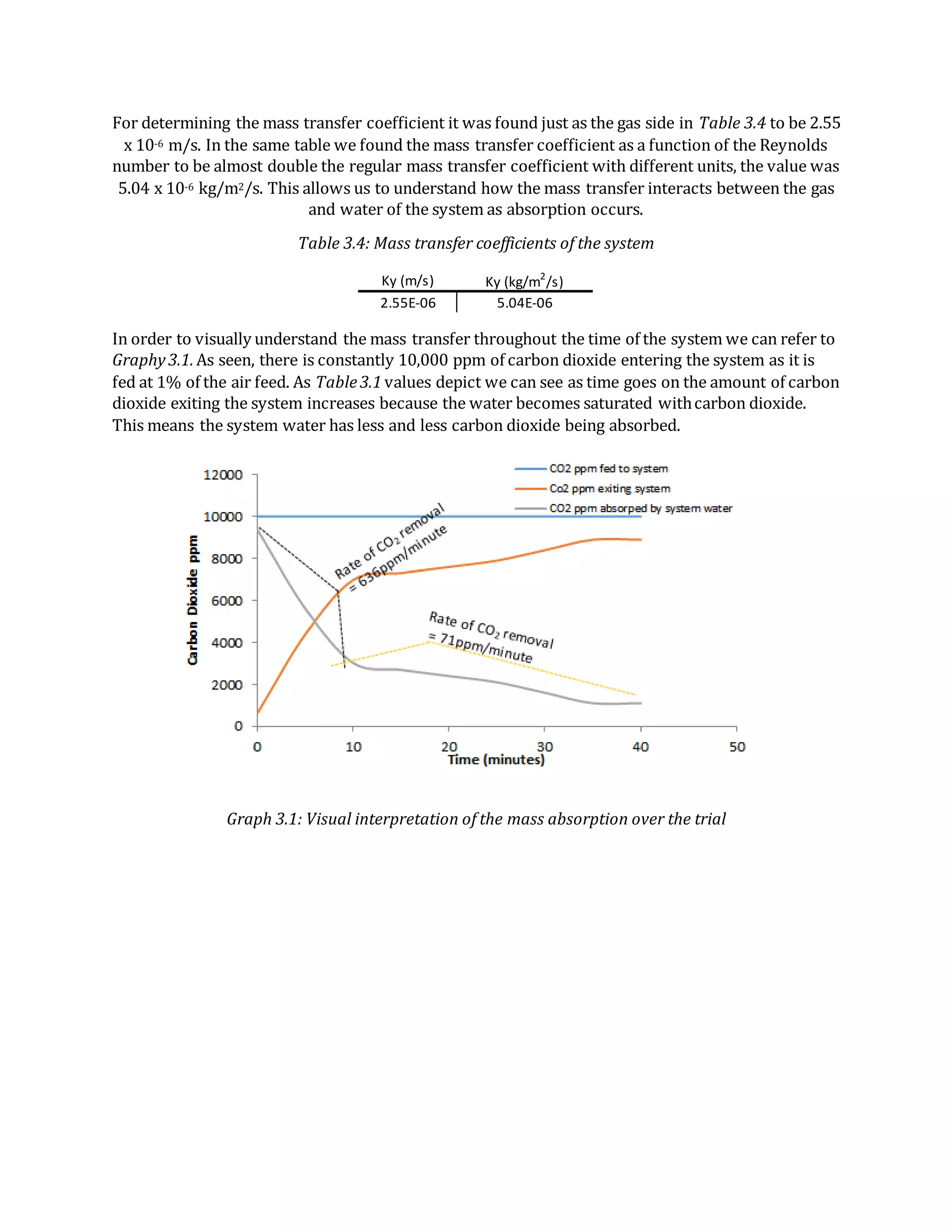
- Absorption of CO2 from air into water was also studied. Data showed decreasing CO2 removal over time as the water became saturated.


![air density is approximately 1.184 kg/m3 or 0.001184 kg/L. The diameter of the circulartube is 80
mm or 0.2625 ft. This results in a cross sectional area of 0.0541 ft2.The equation used:
𝐺𝑦 = (
𝐴𝑖𝑟 𝐹𝑙𝑜𝑤𝑟𝑎𝑡𝑒 𝐿
𝑠
) (
0.001184 𝑘𝑔
𝐿
) (
2.205 𝑙𝑏
𝑘𝑔
) (
1
0.0514 𝑓𝑡2
) = 𝑀𝑎𝑠𝑠 𝐹𝑙𝑜𝑤𝑟𝑎𝑡𝑒 [
𝑙𝑏
𝑠 𝑓𝑡2
]
This is the same method to calculate the mass flow rate of water which has a density of 1 kg/L.
𝐺𝑥 = (
𝑊𝑎𝑡𝑒𝑟 𝐹𝑙𝑜𝑤𝑟𝑎𝑡𝑒 𝐿
𝑠
) (
1 𝑘𝑔
𝐿
) (
2.205 𝑙𝑏
𝑘𝑔
)(
1
0.0514 𝑓𝑡2
) = 𝑀𝑎𝑠𝑠 𝐹𝑙𝑜𝑤𝑟𝑎𝑡𝑒 [
𝑙𝑏
𝑠 𝑓𝑡2
]
Forming the first relationship, ln(Gy) willrepresent the x-axis of the graphical relationship. The y-
axis is the pressure drop on a logarithmic scale. This is calculated by:
𝑙𝑛 (
𝑃ℎ𝑖𝑔ℎ − 𝑃𝑙𝑜𝑤
∆𝐻
)
This is the difference in pressures divided by the distance between the reading points in the tower.
Creating a generalized correlation would require many data points relating the flow topressure
drop. From the worksheet, there were twoequations identified to represent the x and y axes for
constructing the generalized correlations. They are as follows.
𝑥 − 𝑎𝑥𝑖𝑠:
𝐺𝑥
𝐺𝑦
√
𝜌𝑦
𝜌𝑥
𝑦 − 𝑎𝑥𝑖𝑠:
𝐺𝑦
2 𝐹𝑝 (
62.3
𝜌𝑥
) 𝜇 𝑥
0.2
𝑔 𝑐 𝜌𝑥 𝜌𝑦
Where
𝐹𝑝 − 𝐹𝑙𝑜𝑜𝑑 𝑃𝑜𝑖𝑛𝑡 (= 1024 𝑓𝑡−1)
𝑔 𝑐 − 𝑝𝑟𝑜𝑝𝑜𝑟𝑡𝑖𝑜𝑛𝑎𝑙𝑖𝑡𝑦 𝑓𝑎𝑐𝑡𝑜𝑟 (= 32.174
𝑓𝑡 𝑙𝑏
𝑙𝑏𝑓 𝑠2
)
𝜇 𝑥 − 𝐿𝑖𝑞𝑢𝑖𝑥 𝑣𝑖𝑠𝑐𝑜𝑠𝑖𝑡𝑦 (8.90𝐸 − 4
𝑘𝑔
𝑠 𝑚
)
The relationship between these twoparameters wouldform the generalized correlation.](https://image.slidesharecdn.com/absorption-190314072956/75/On-Absorption-Practice-Methods-and-Theory-An-Empirical-Example-3-2048.jpg)
![To constructthe generalized correlation, a graph of the data points was generated at first. This is
shown as Graph 2.2 with each data set corresponding to a different water flowrate. The axes are
described in the experimental development section and willbe restated.
𝑥 − 𝑎𝑥𝑖𝑠:
𝐺𝑥
𝐺𝑦
√
𝜌𝑦
𝜌𝑥
𝑦 − 𝑎𝑥𝑖𝑠:
𝐺𝑦
2 𝐹𝑝 (
62.3
𝜌𝑥
) 𝜇 𝑥
0.2
𝑔 𝑐 𝜌𝑥 𝜌𝑦
Eachpoint represents a different pressure drop relation. Again, the pressure drop is calculated
with respect to the total height difference. The units are also important as well.
𝑃𝑟𝑒𝑠𝑠𝑢𝑟𝑒 𝐷𝑟𝑜𝑝 =
𝑃ℎ𝑖𝑔ℎ − 𝑃𝑙𝑜𝑤
ℎ𝑒𝑖𝑔ℎ𝑡
[
𝑖𝑛 𝐻2 𝑂
𝑓𝑡
]
To constructthe correlation, points with similar pressure drops werefocused on and assessed for
the accuracy of the regression. This is shown in Graph 2.3.
Graph 2.3: Linear regression of pressure drops. See Table 2.1 for the parameters of each line moving from the
top line downward.
These regressions were significantly accurate and provided a basis forthe graphical construction.
The R2 values were all above 0.95 confirming the accuracy of the measurements. The linear
regression was accurateenough to remain the desired correlation method (as opposed to
exponential or other). The results are shown in Table 2.1 withthe slope of each line, the intercept,
the pressure drop value corresponding to the line, and the R2 value. The table is ordered so that
each column position relates to the position of each line in the graph, highest being first, lowest
being last. For example, the “floodpoint” line is the uppermost line on the graph. “Line 5” is the
lowermost line on the graph.
17.5
18
18.5
19
19.5
20
20.5
-9 -8.5 -8 -7.5 -7 -6.5 -6 -5.5
ln(y)
ln(x)](https://image.slidesharecdn.com/absorption-190314072956/75/On-Absorption-Practice-Methods-and-Theory-An-Empirical-Example-5-2048.jpg)
![Table 3.1: ppm Balance of the absorption tower
In Table3.2 a very similar balance is done but instead of ppm the numbers have been converted
into moles of carbon dioxide in water.Similar explanations and ideas revolvearound this table as
they do for the discussion of Table3.1. Using this table we can determine that roughly 0.035 moles
of carbon dioxide are now in the water.
Table 3.2: mole balance of the Absorption Tower
Here in Table3.3 we can observe how the rate of carbon dioxide removal from the gas phase by
absorption in water changes throughout the experiment. For the first ten minutes the rate of
removal was very high and efficientat around 640 ppm/min of carbonremoved fromgas phase.
After ten minutes the water became toosaturated and the rate of removal dropped to 71 ppm/min.
The last removal rate that we lookedat is the bold number whichdetermines the rate over the
entire forty minute period, we found that the overall rate of carbon dioxide removal from the gas
phase by absorption by water was 207 ppm/min.
Table 3.3: Rate of Removal of Carbon Dioxide
Time (min) pH (z)
CO2 ppm fed to
system
Co2 ppm exiting
system
CO2 ppm
absorped by
system water
0 10.5 10000 617 9383
5 10.5 10000 4381 5619
10 10.1 10000 6980 3020
15 10.1 10000 7310 2690
20 9.9 10000 7600 2400
25 9.8 10000 7900 2100
30 9.7 10000 8400 1600
35 9.7 10000 8900 1100
40 9.5 10000 8900 1100
Time (min) pH (z) Exit CO2 mol frac Exit CO2 moles/min [H+] ions Ct (moles/L)
Moles
CO2 in
water
CO2
Moles
leaving
0 10.5 0.0006 0.0013 3.16E-11 -1.14E-06 0.0000 0.0000
5 10.5 0.0044 0.0090 3.16E-11 -1.14E-06 0.0000 0.0448
10 10.1 0.0070 0.0143 7.94E-11 1.10E-04 0.0031 0.1429
15 10.1 0.0073 0.0150 7.94E-11 1.10E-04 0.0031 0.2244
20 9.9 0.0076 0.0156 1.26E-10 1.62E-04 0.0046 0.3111
25 9.8 0.0079 0.0162 1.58E-10 1.85E-04 0.0053 0.4043
30 9.7 0.0084 0.0172 2.00E-10 2.05E-04 0.0059 0.5158
35 9.7 0.0089 0.0182 2.00E-10 2.05E-04 0.0059 0.6376
40 9.5 0.0089 0.0182 3.16E-10 2.39E-04 0.0068 0.7287
Time
Range
(min)
Rate of
Removal
(ppm/min)
0 - 10 636
10 - 40 71
0 - 40 207](https://image.slidesharecdn.com/absorption-190314072956/75/On-Absorption-Practice-Methods-and-Theory-An-Empirical-Example-8-2048.jpg)
![Table 4: Tabulation of back calculated values for the addition on NaOH to raise pH from 8.3
to 10.3
Additional Notes and Further Inquiry:
The back calculationof the mass of NaOH needed to be added to our system to raise the pH
by three points was rather straightforward. It also yielded results that were pretty close to the
actual, known value of NaOH added to the system. The principal behind this calculation was that pH
is based off of molarity, whichis a concentration measurement. The pH gave us a moles/liter value.
As shown above,we calculated these values. From there, we knew that there was about 28.5 liters
of water solution in out system. This allowed us to multiply the difference in molarities by the
amount of liters of solution. The stoichiometry of NaOH and H2O is one to one, so a direct molar
correlation could be achieved. This yielded a molar value forNaOH. From there, the molecular
weight was used to yield a final mass value, of about 4.4 grams of NaOH. As noted before, we knew
the amount of NaOH that we added, because we weighed it before the addition. Our measured
weight was about 4.1 grams. This value is very closeto the theoretically calculated value. Some
reasons why it coulddiffer slightly are that while the waterwas flowing,residual acidic media may
have been left overon the walls and parts of the system. Also, human error in calculation probably
played a role in the discrepancy. Overall,we were not very surprised by this calculation and
expected a small error involved.
Please Inquire at lehmanjw@miamioh.edu
Back Calculated Parameters Calculated Value
Molarity of Solution at pH of 8.3 [H+] 5.013 x 10-9 moles/liter
Molarity of Solution at pH of 10.3 [H+] 5.001 x 10-11 moles/liter
Moles of NaOH needed to be added 0.110 moles NaOH
Weight of NaOH to be added (39.9 g/mol) 4.412 grams NaOH](https://image.slidesharecdn.com/absorption-190314072956/75/On-Absorption-Practice-Methods-and-Theory-An-Empirical-Example-10-2048.jpg)
