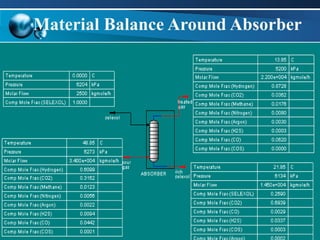
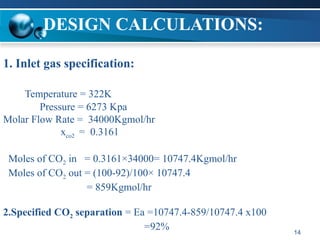
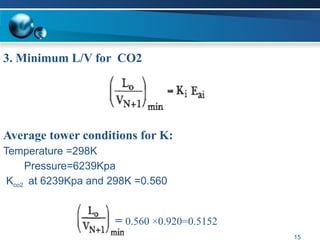
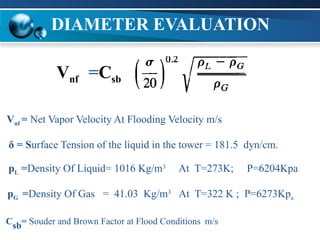
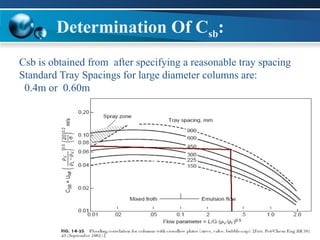
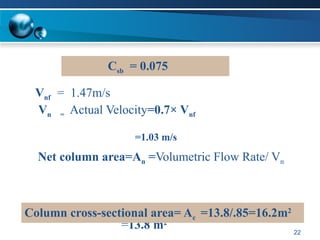
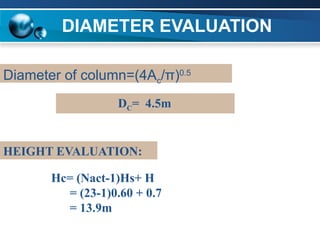
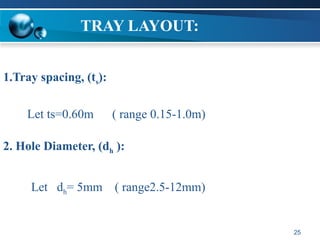
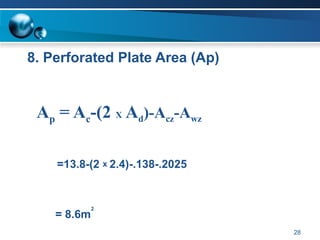
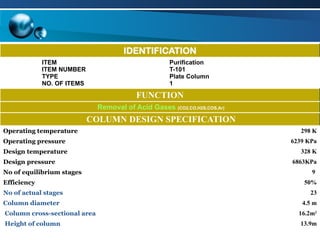
The document outlines the design and operational specifications of an absorber system for gas absorption, focusing on the selection and design of plate columns using Selexol solvent for efficient removal of acid gases. Key features of the Selexol solvent include its low vapor pressure, non-reactivity, and ability to handle high solubility for various contaminants. The design calculations detail the necessary parameters for a successful absorption process, including material balances and column dimensions.