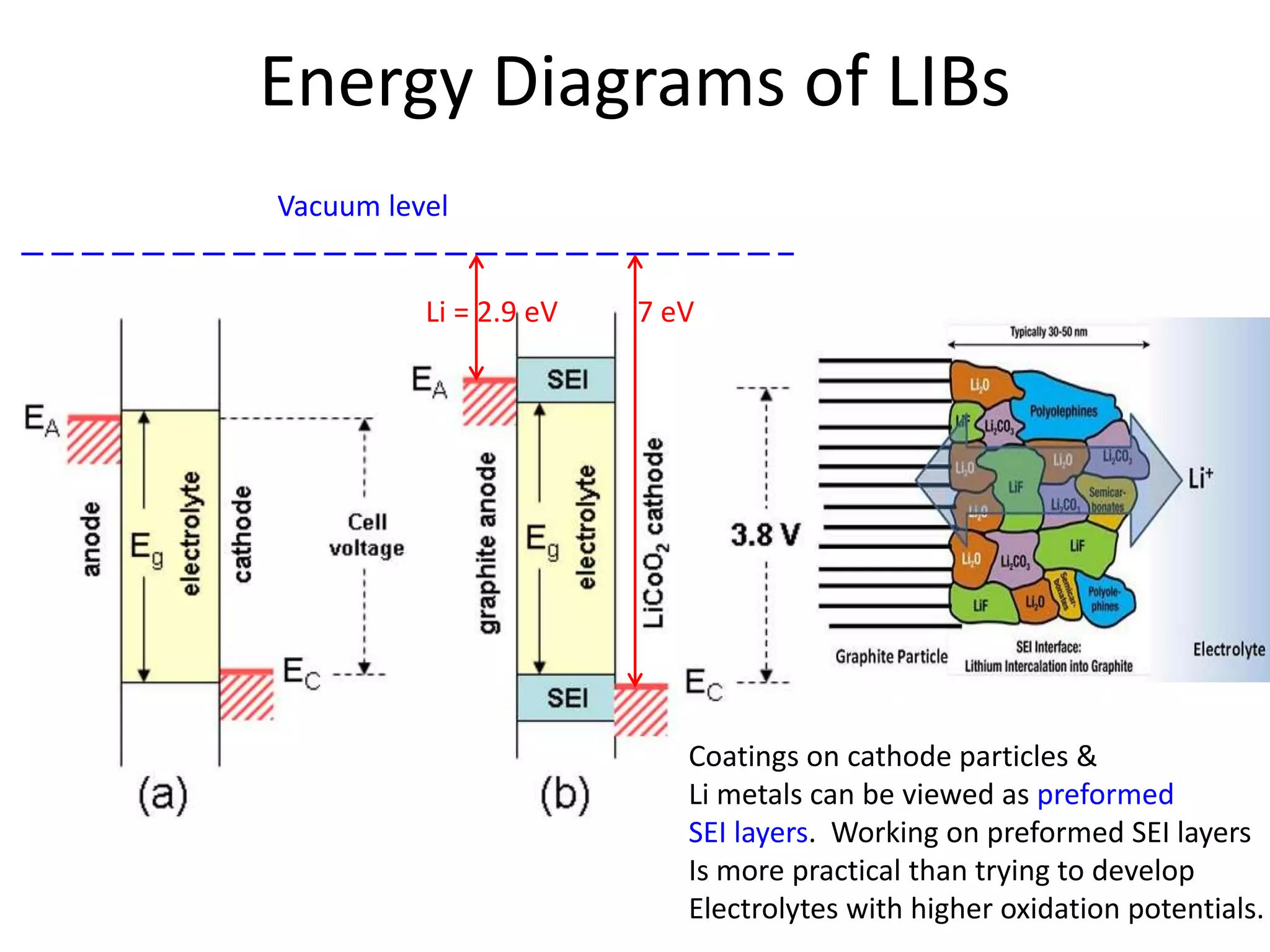
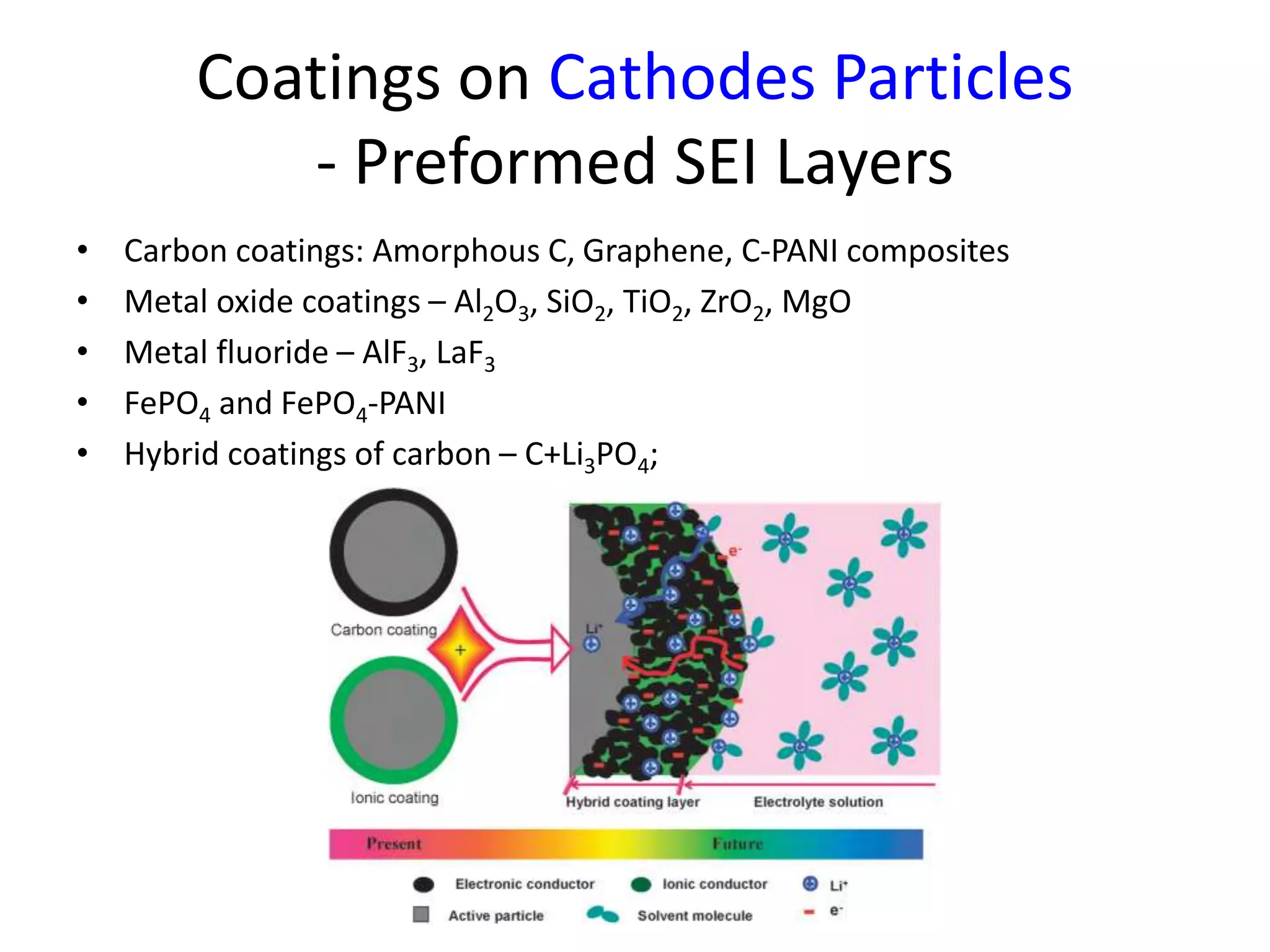
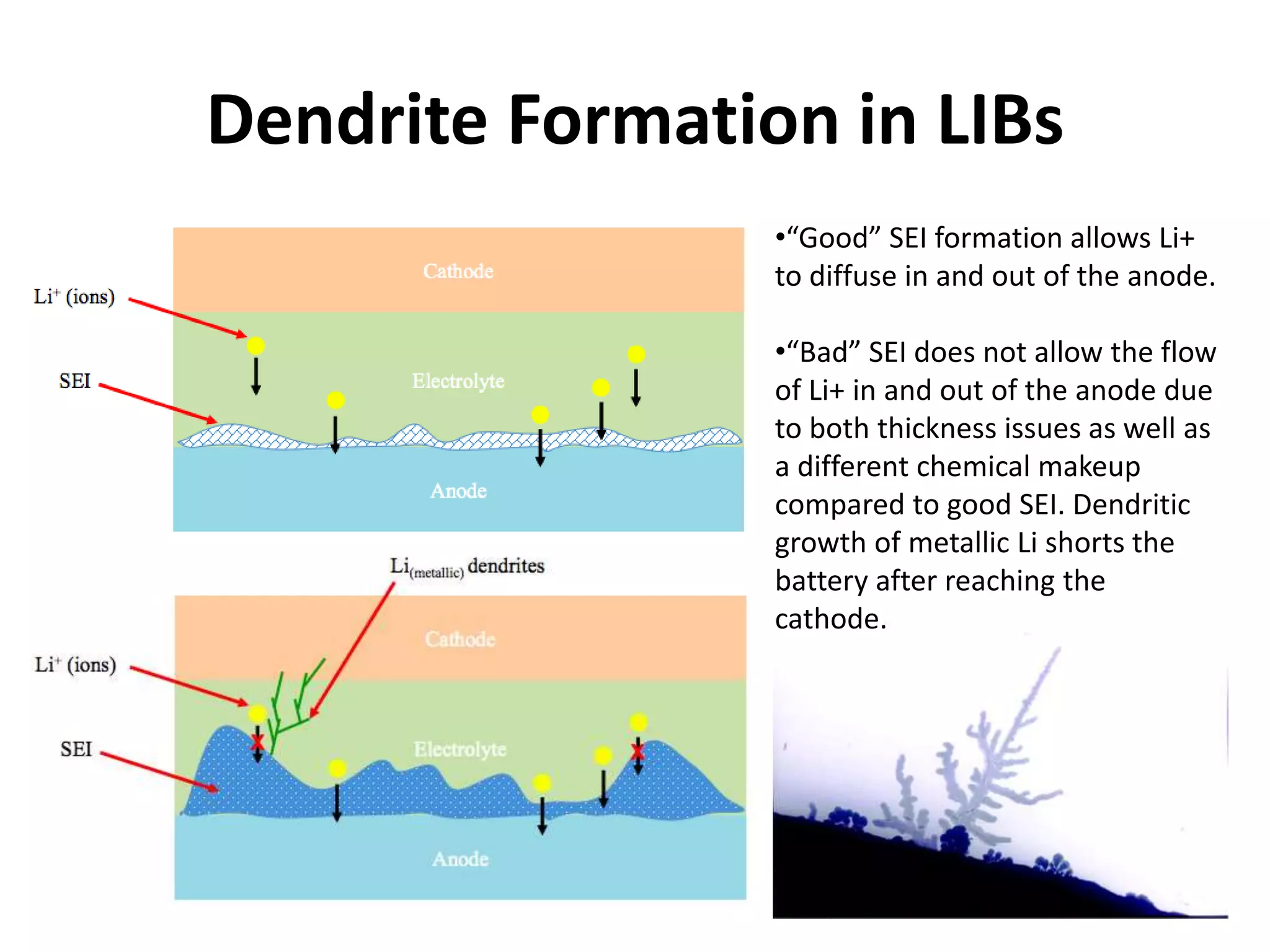
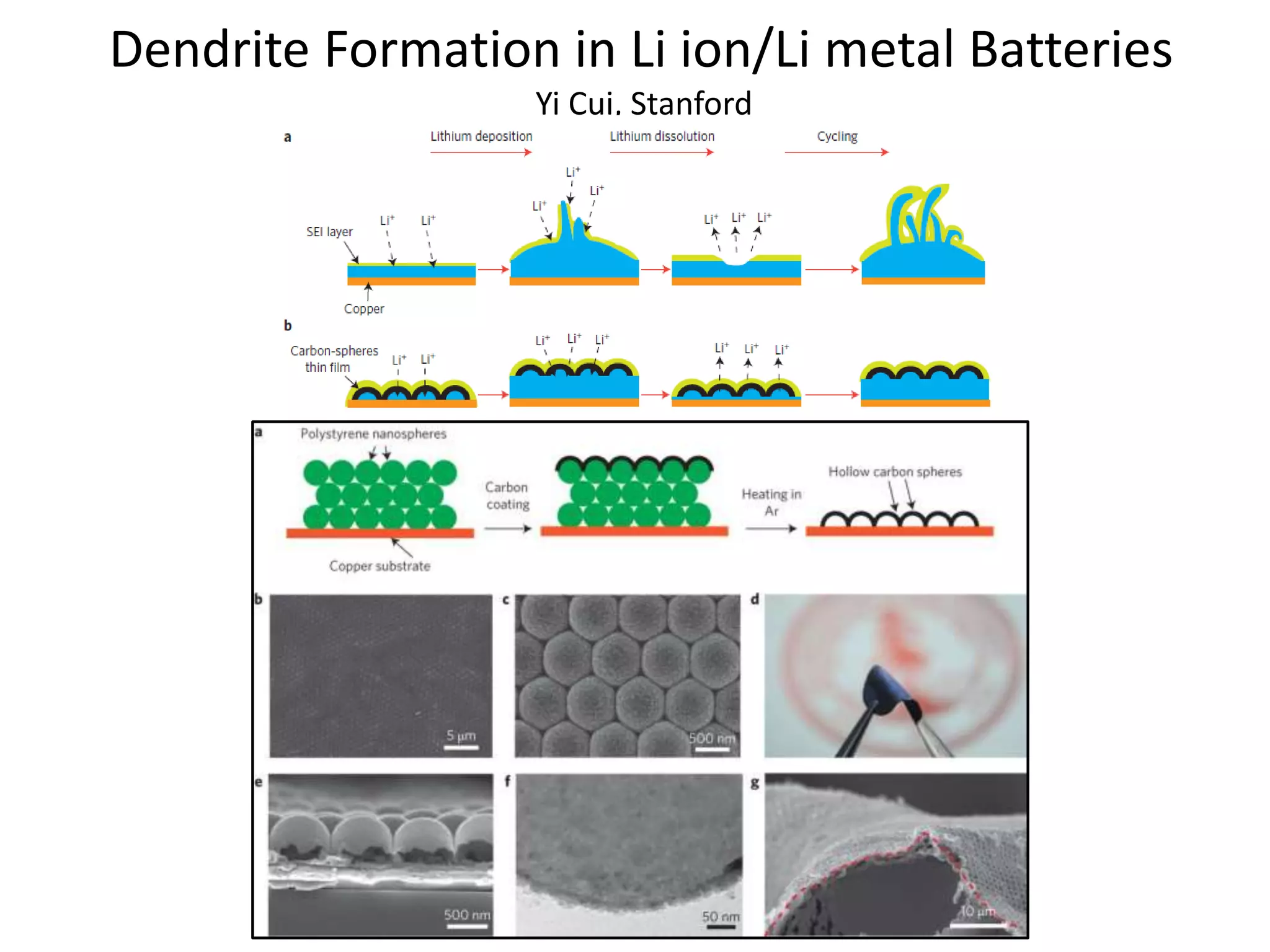
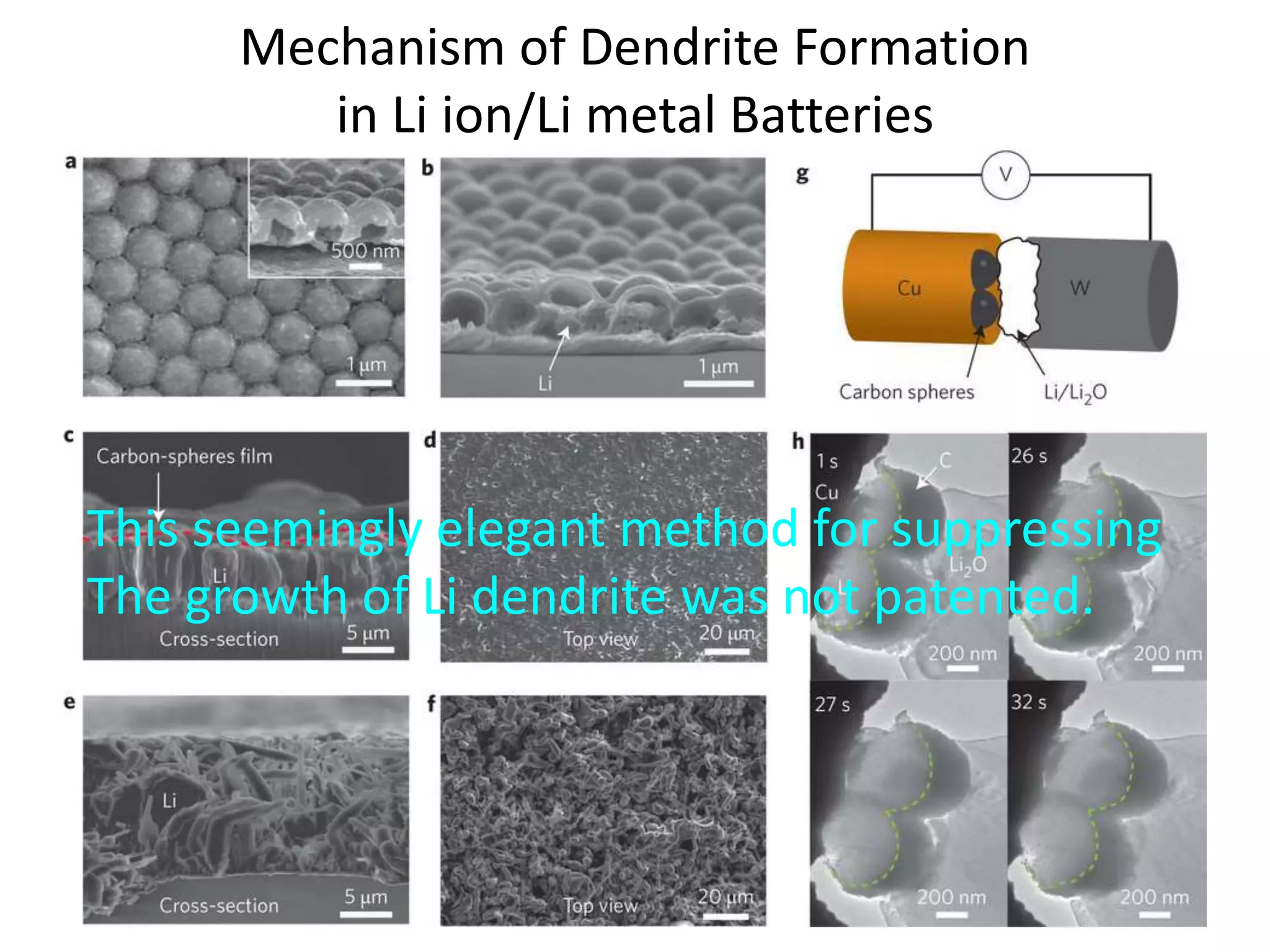
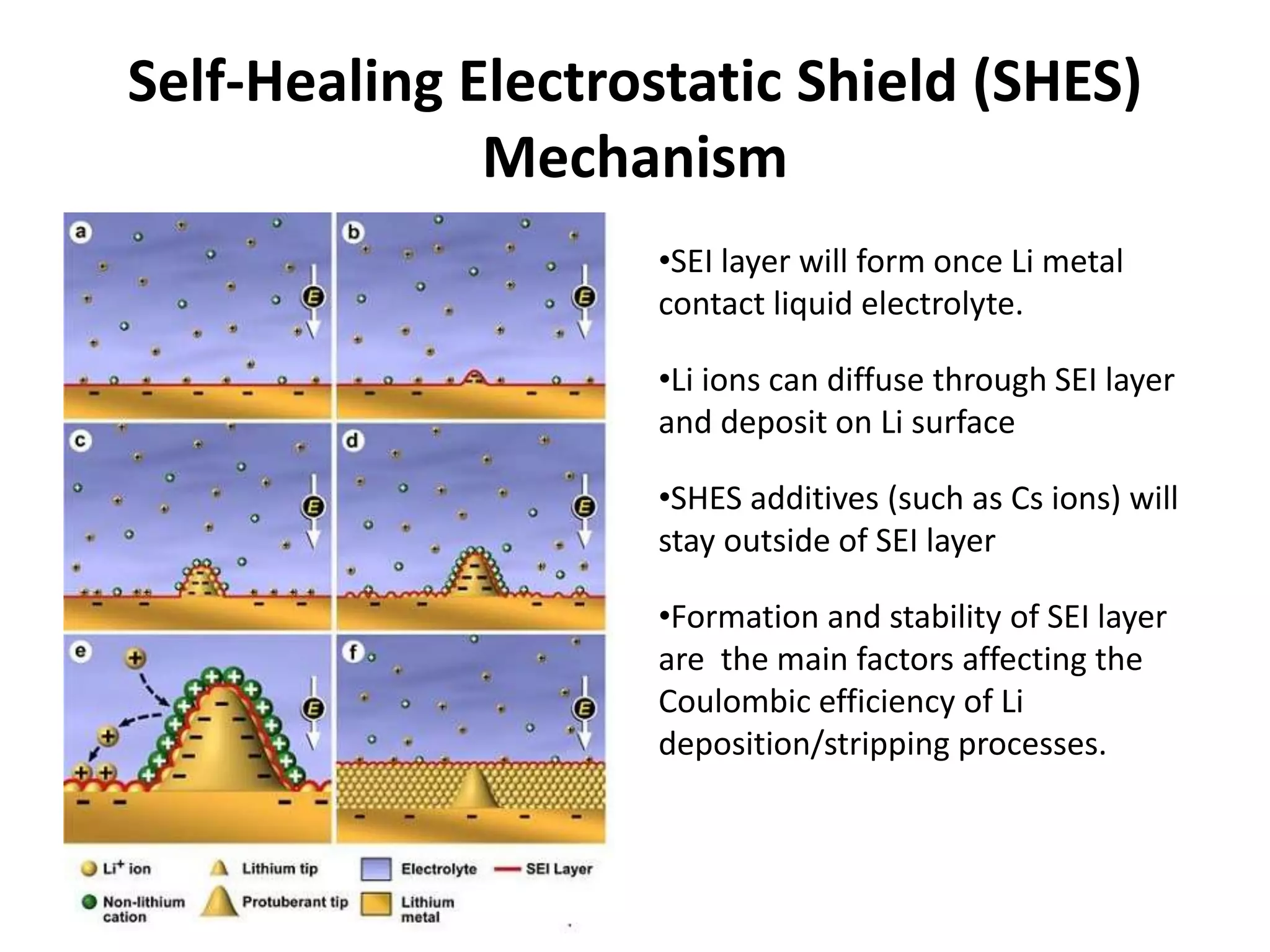
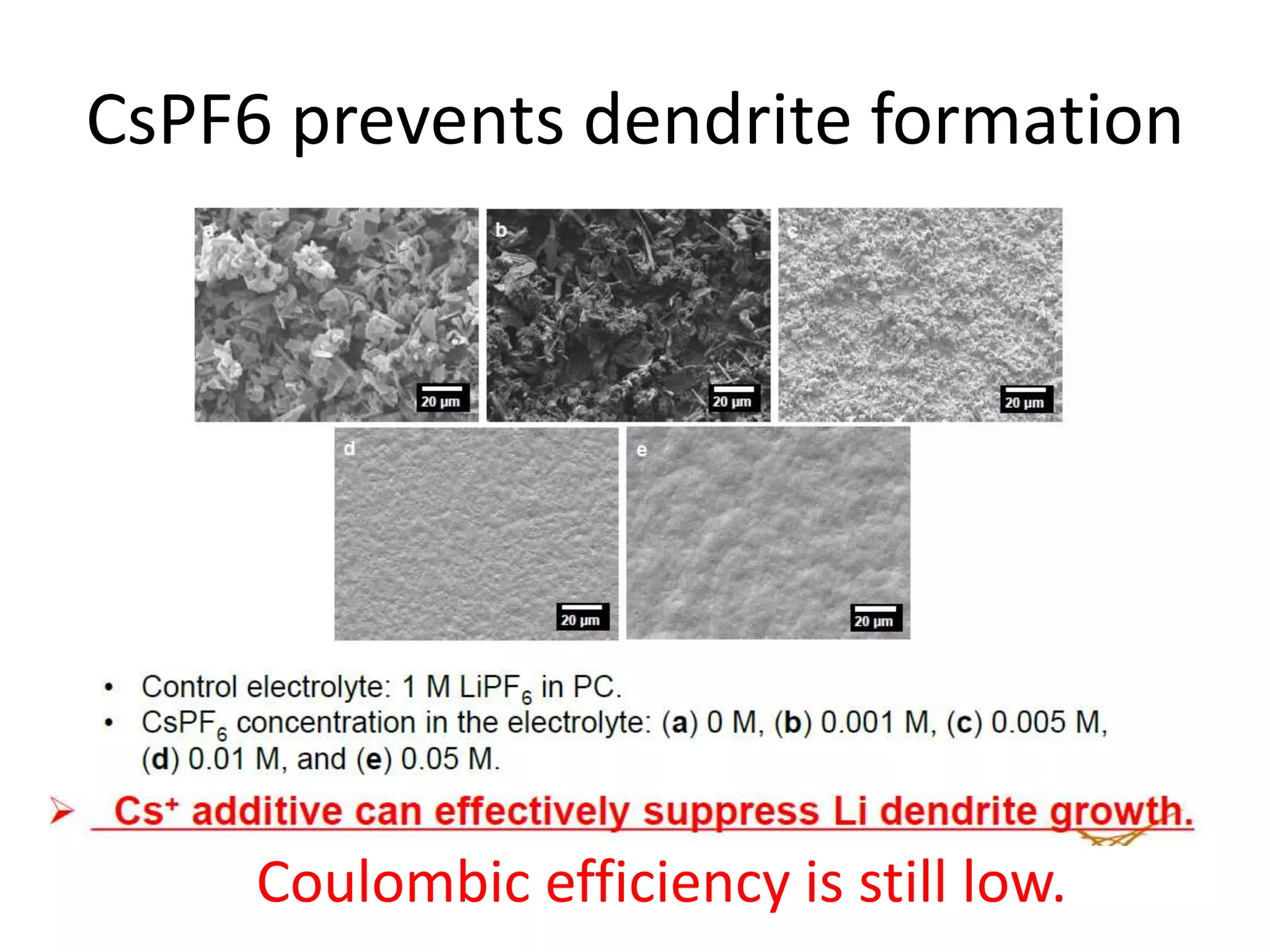
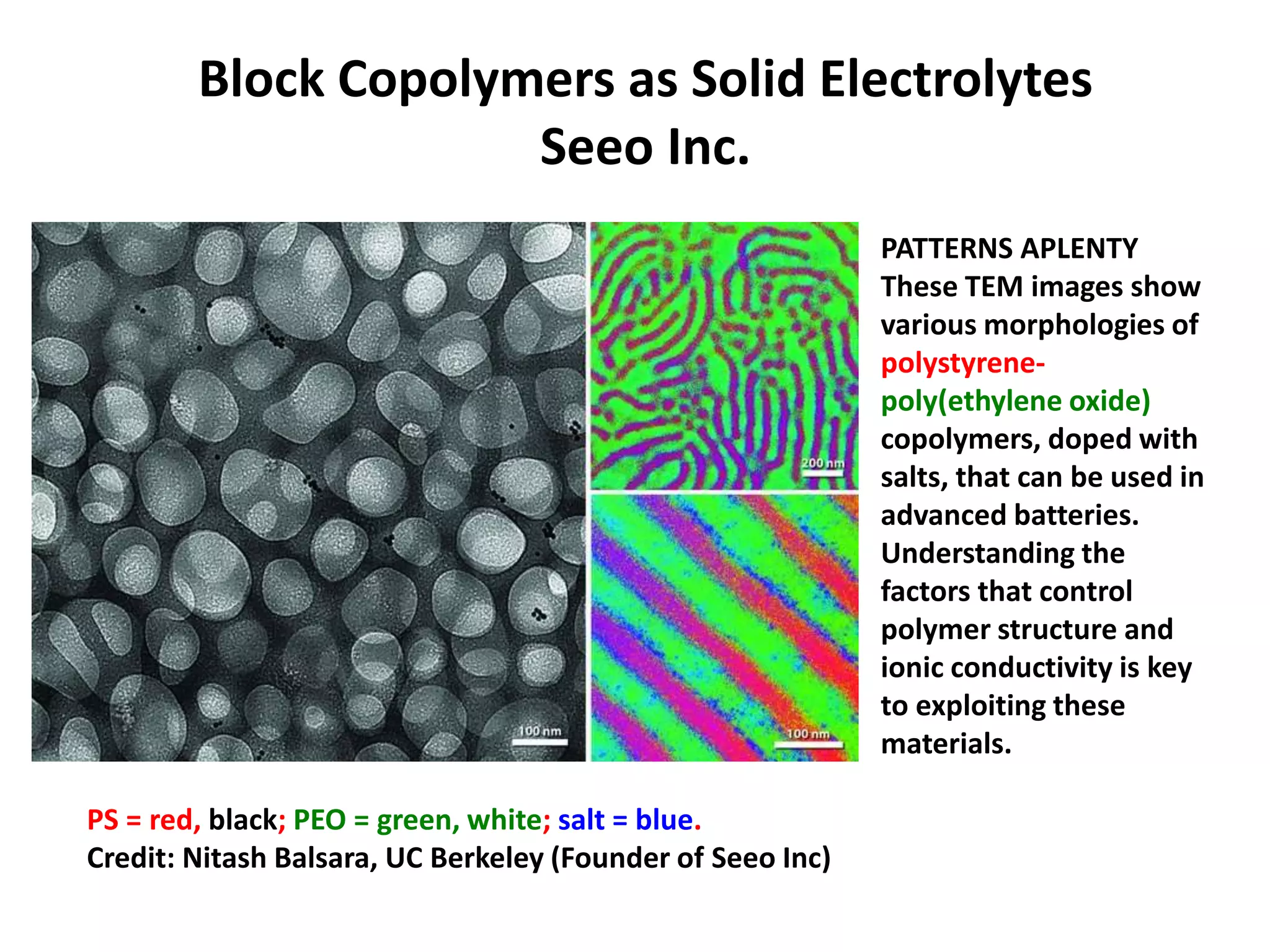
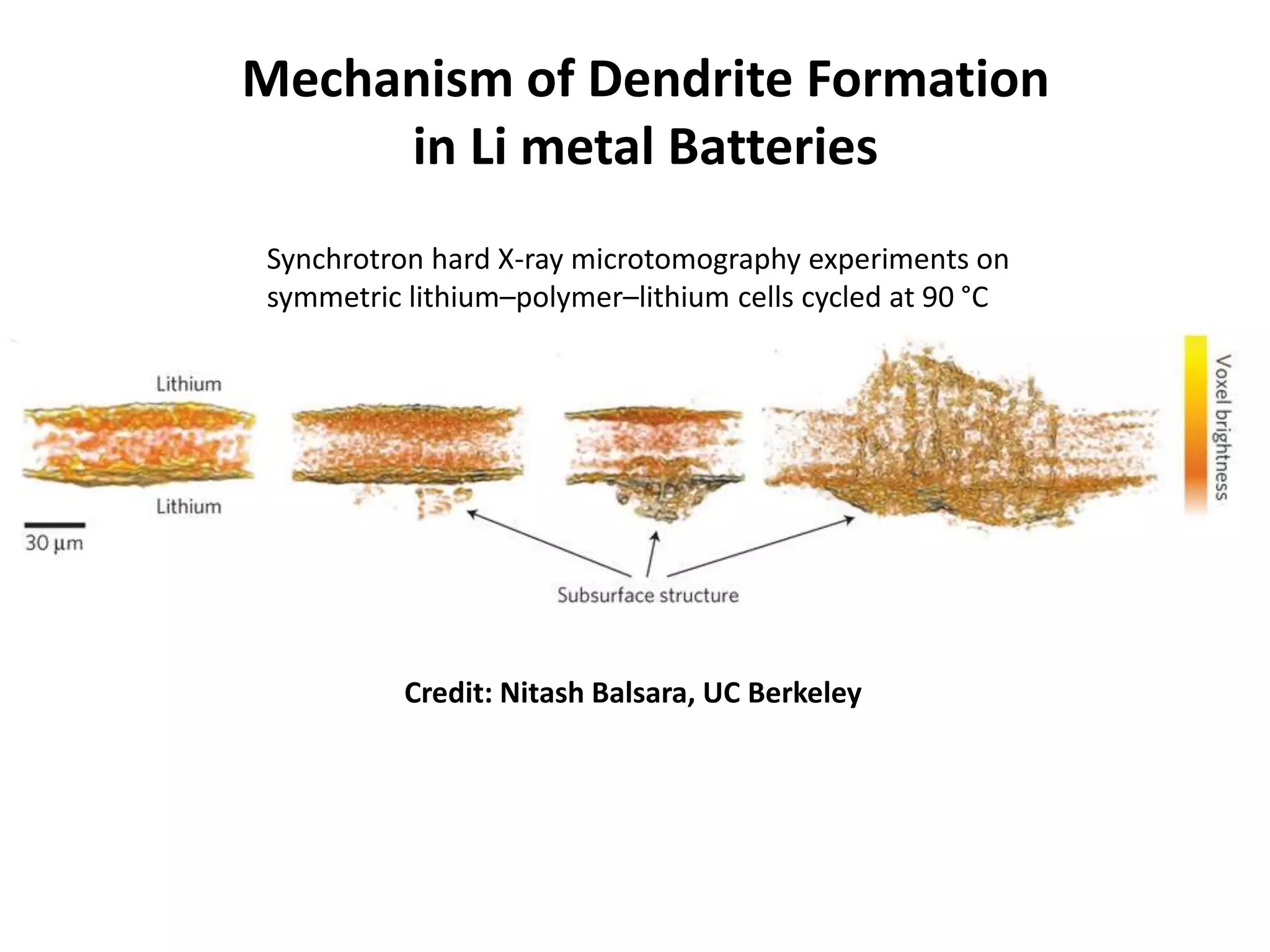
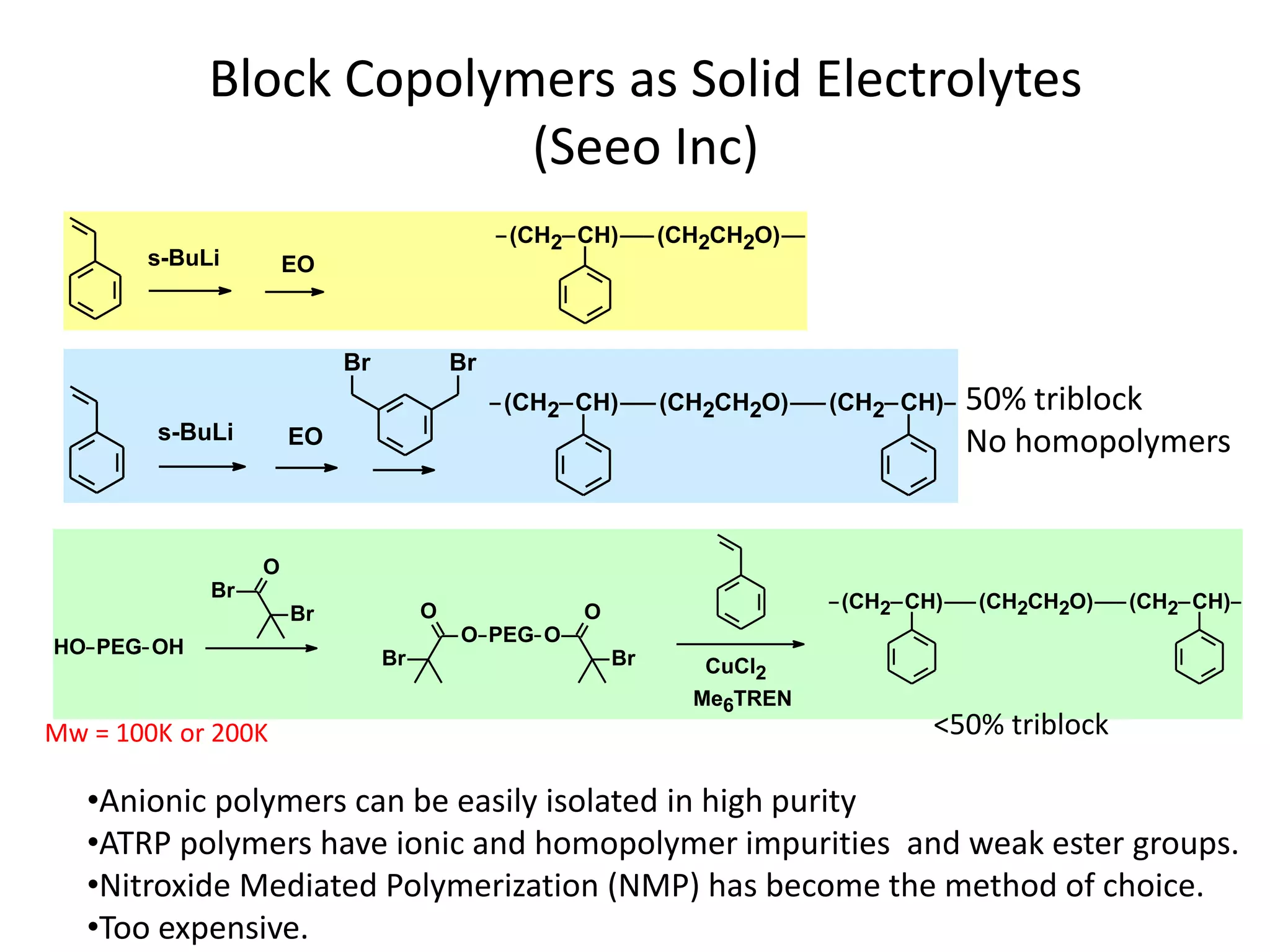
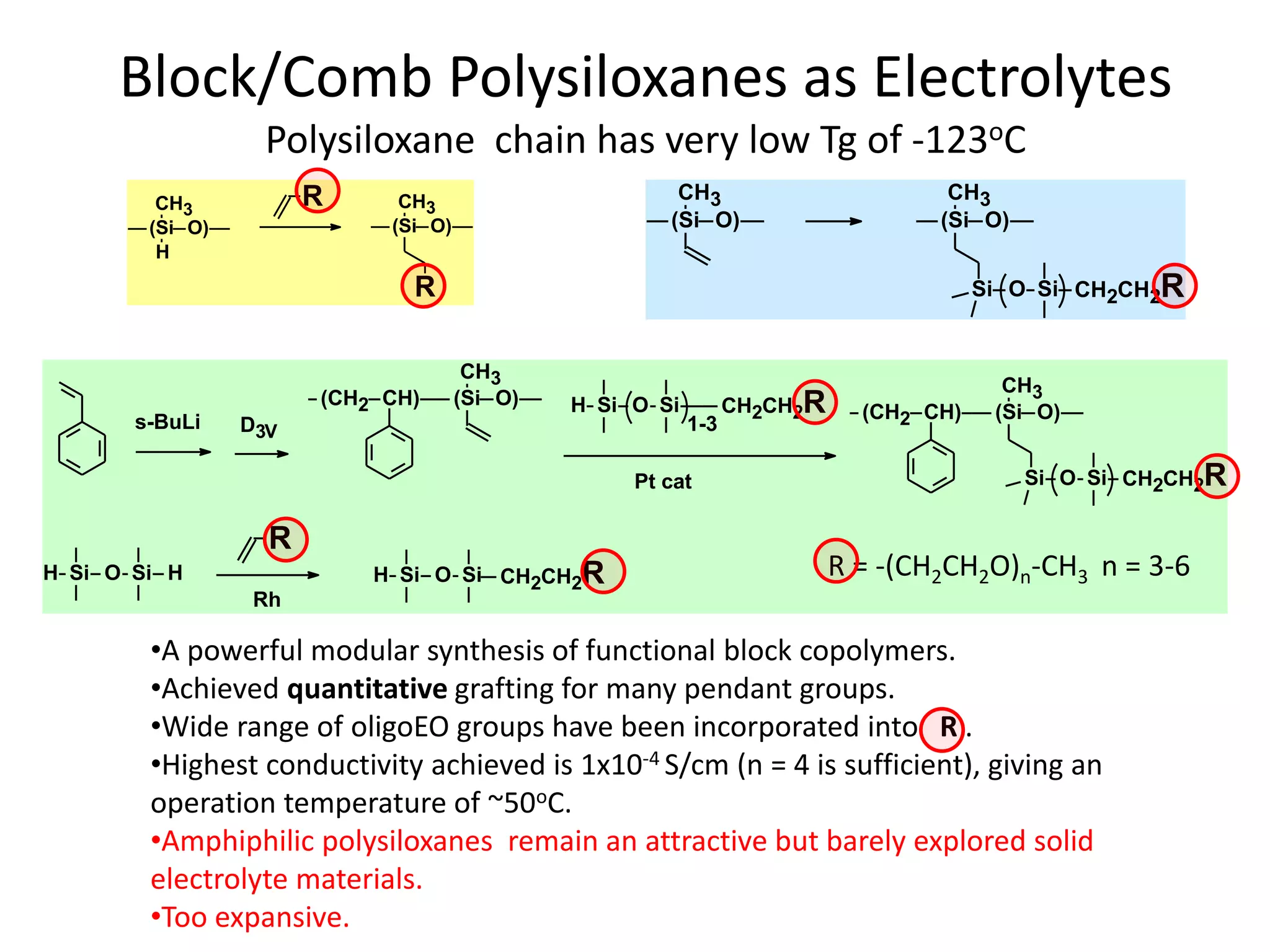
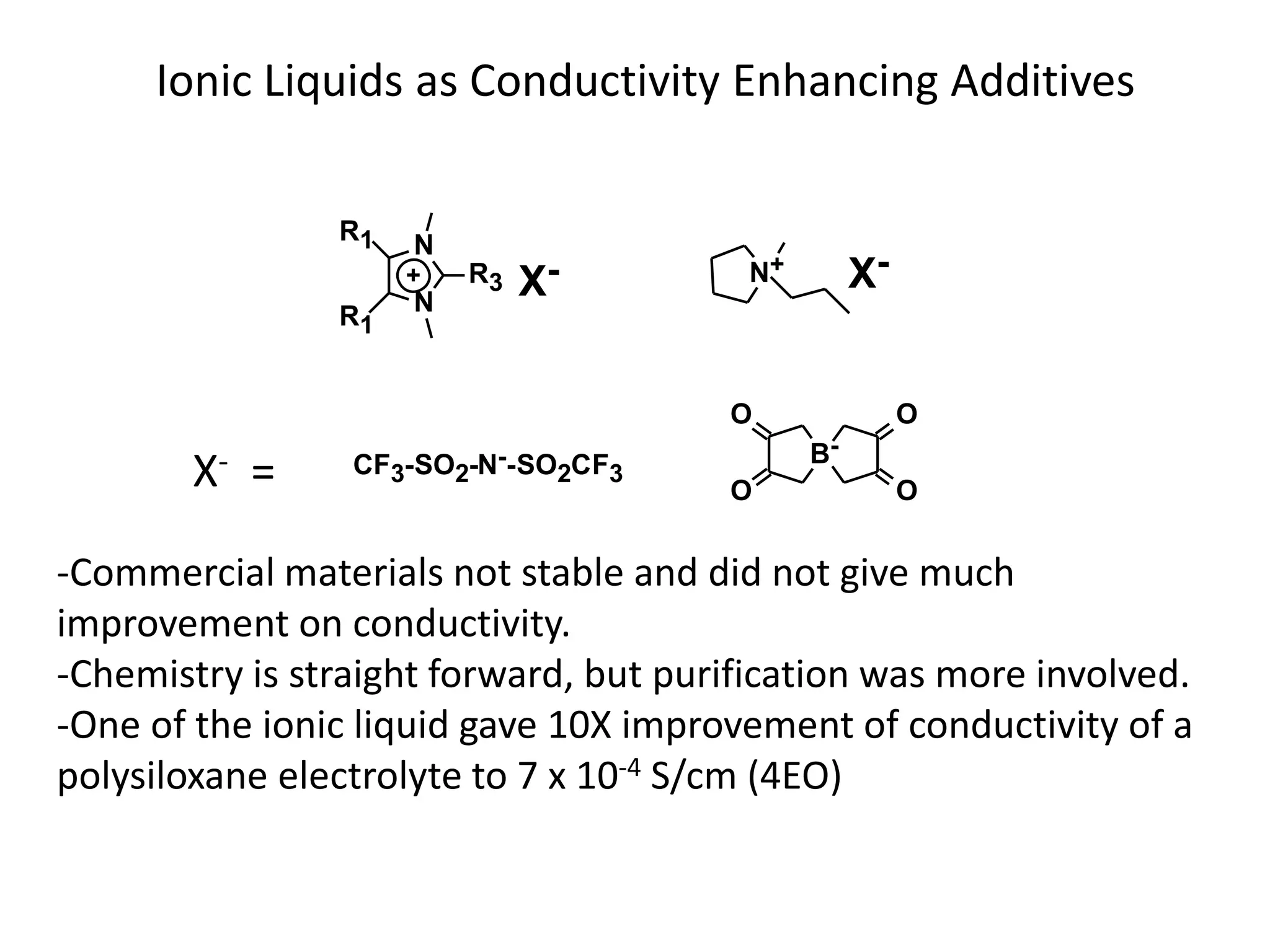
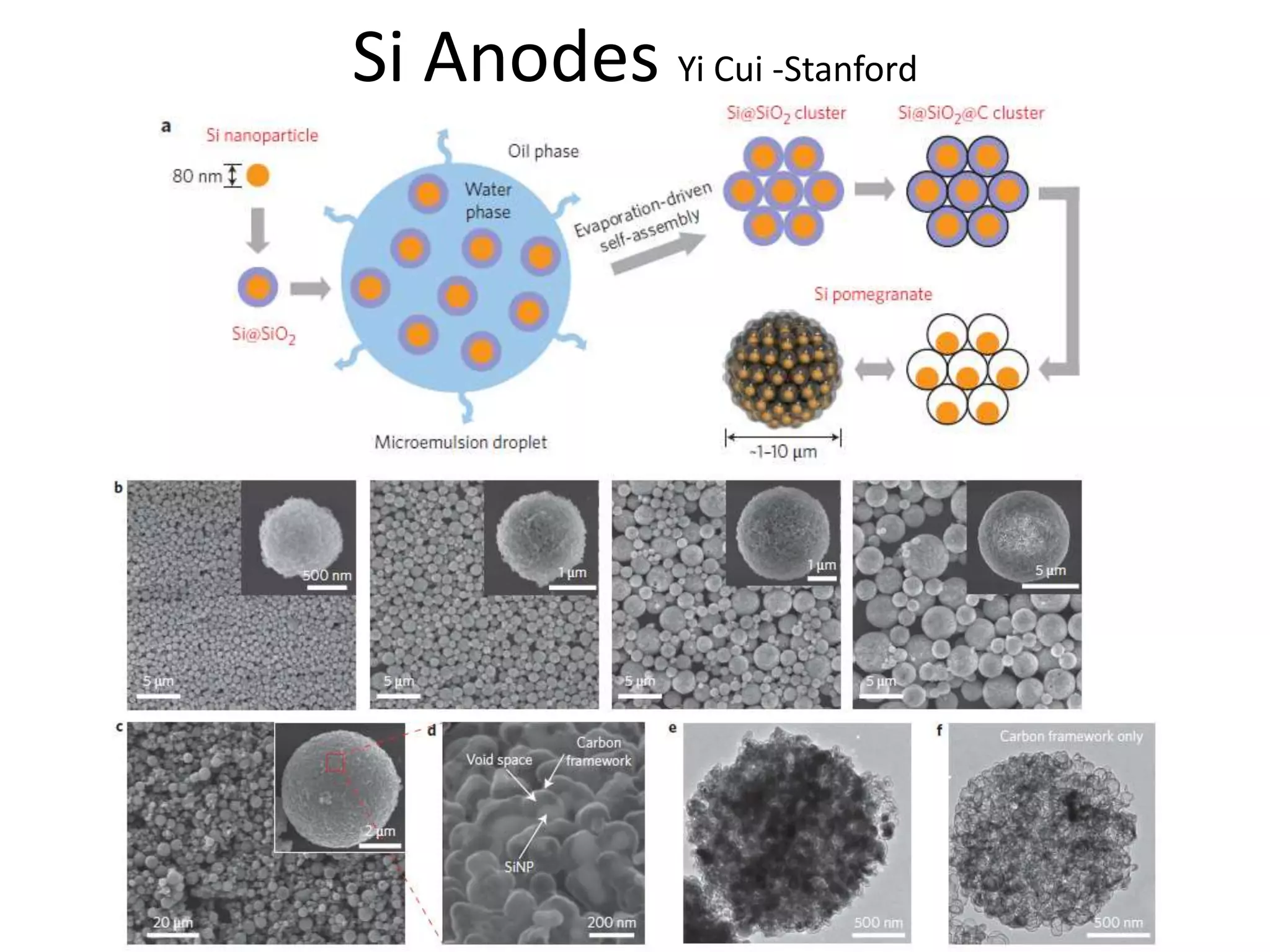
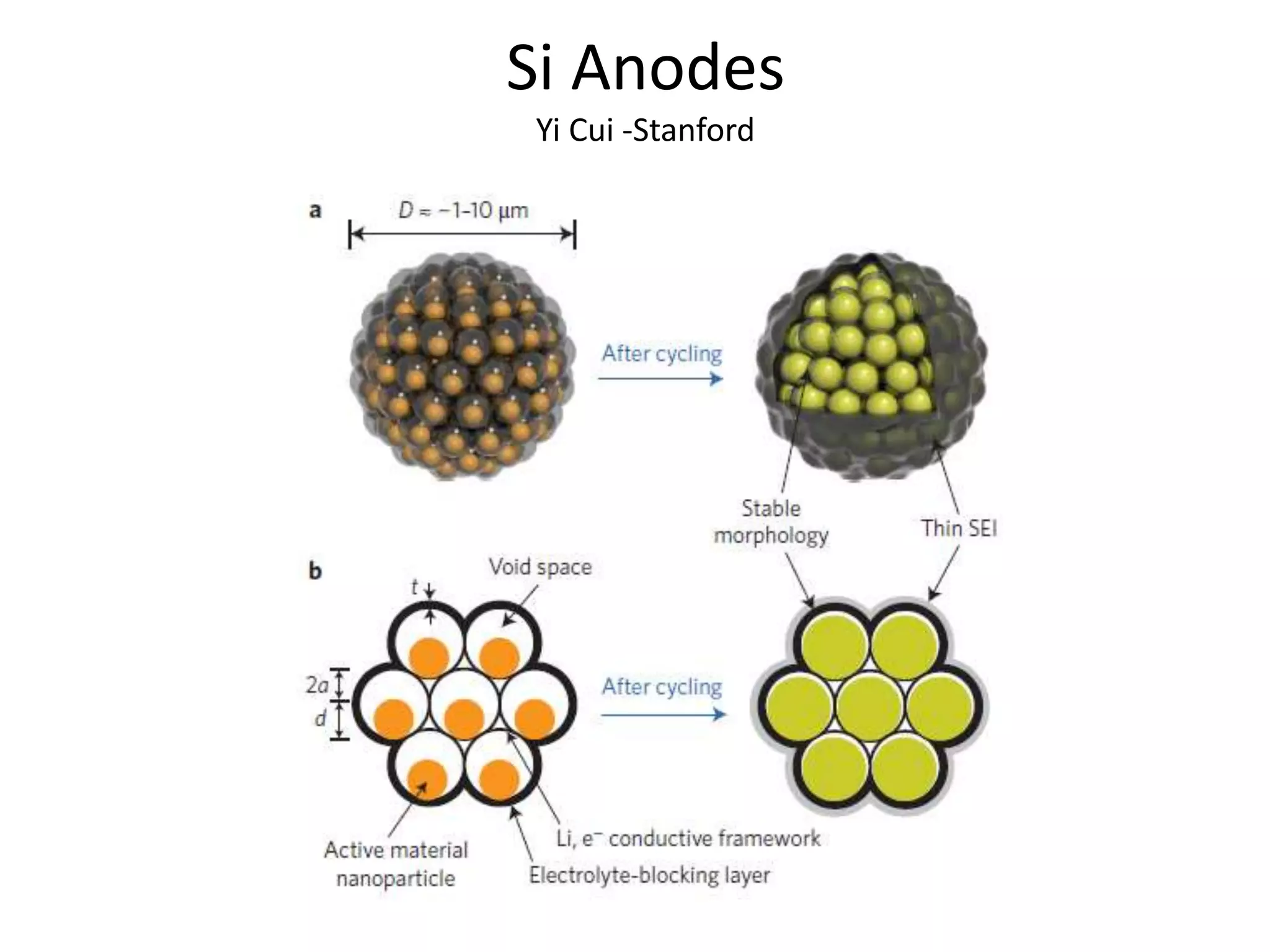
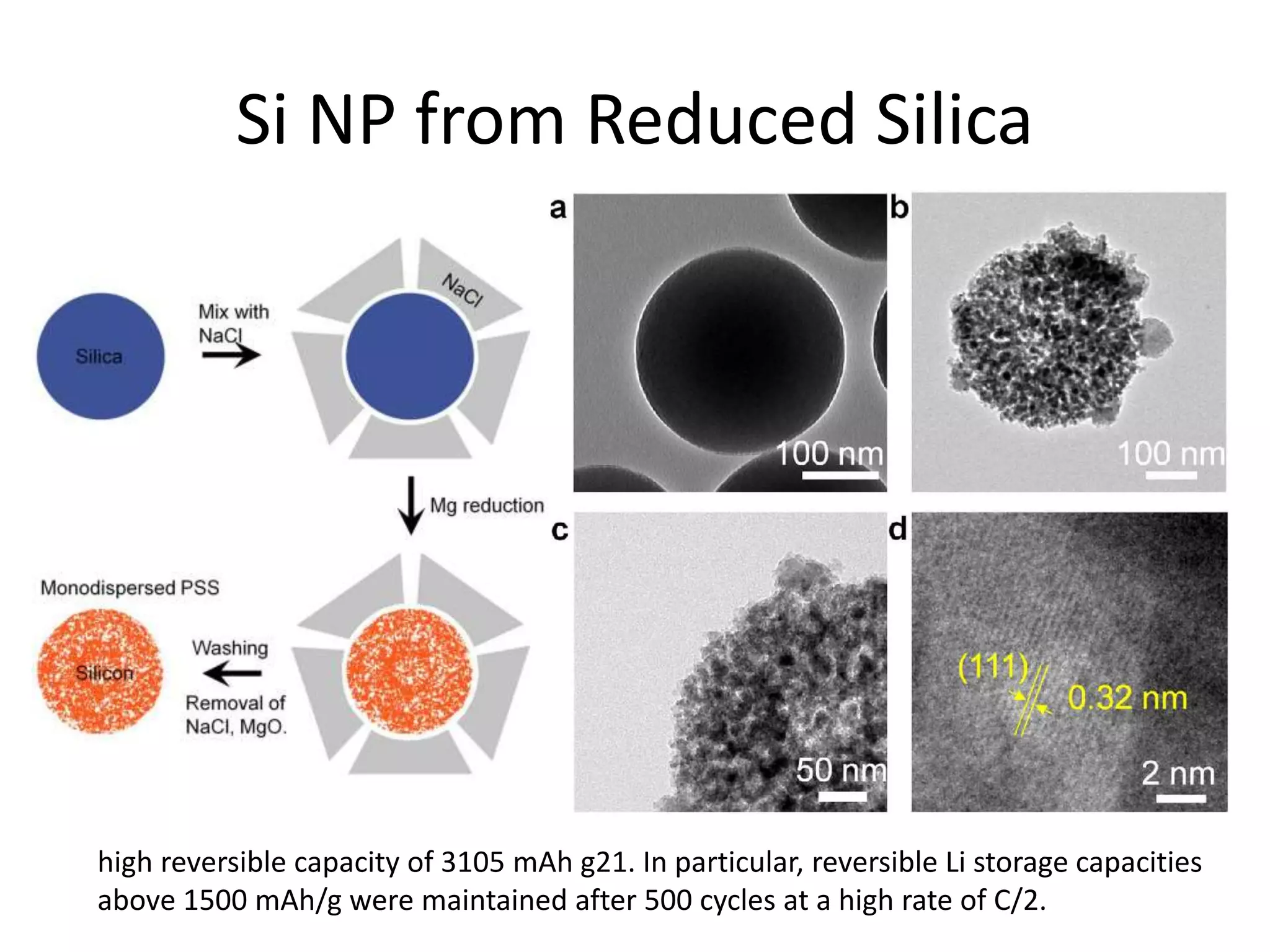
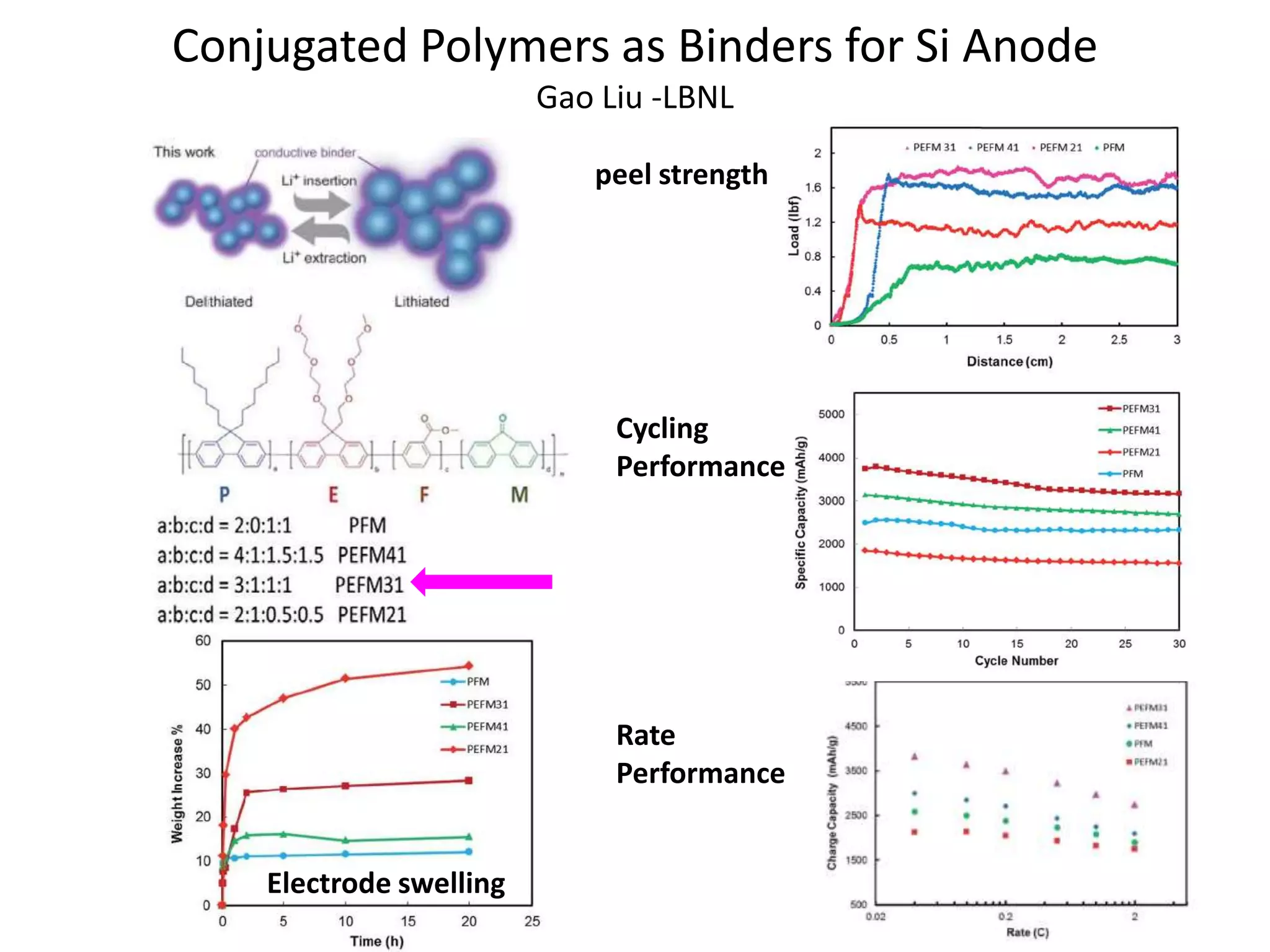
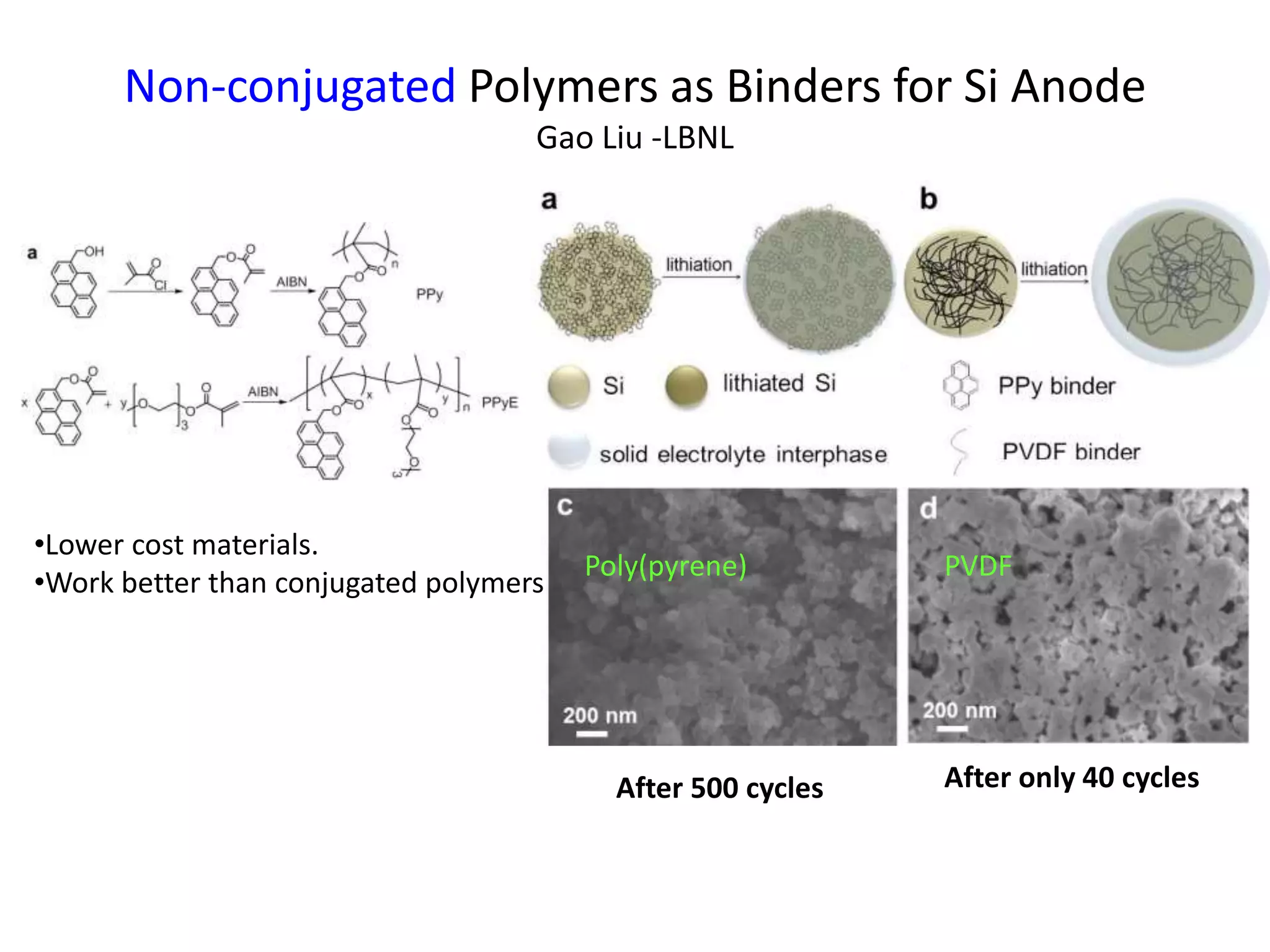
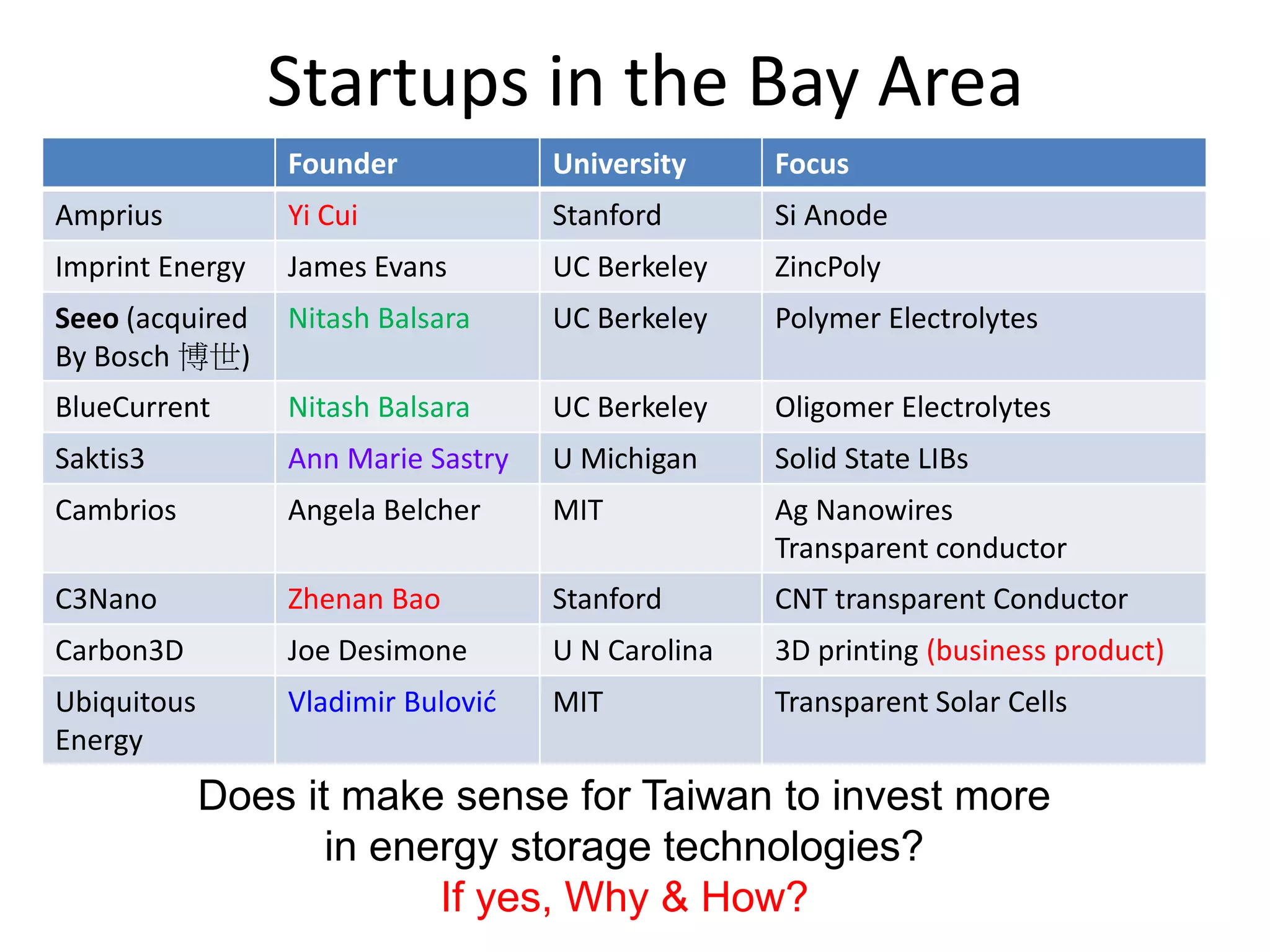
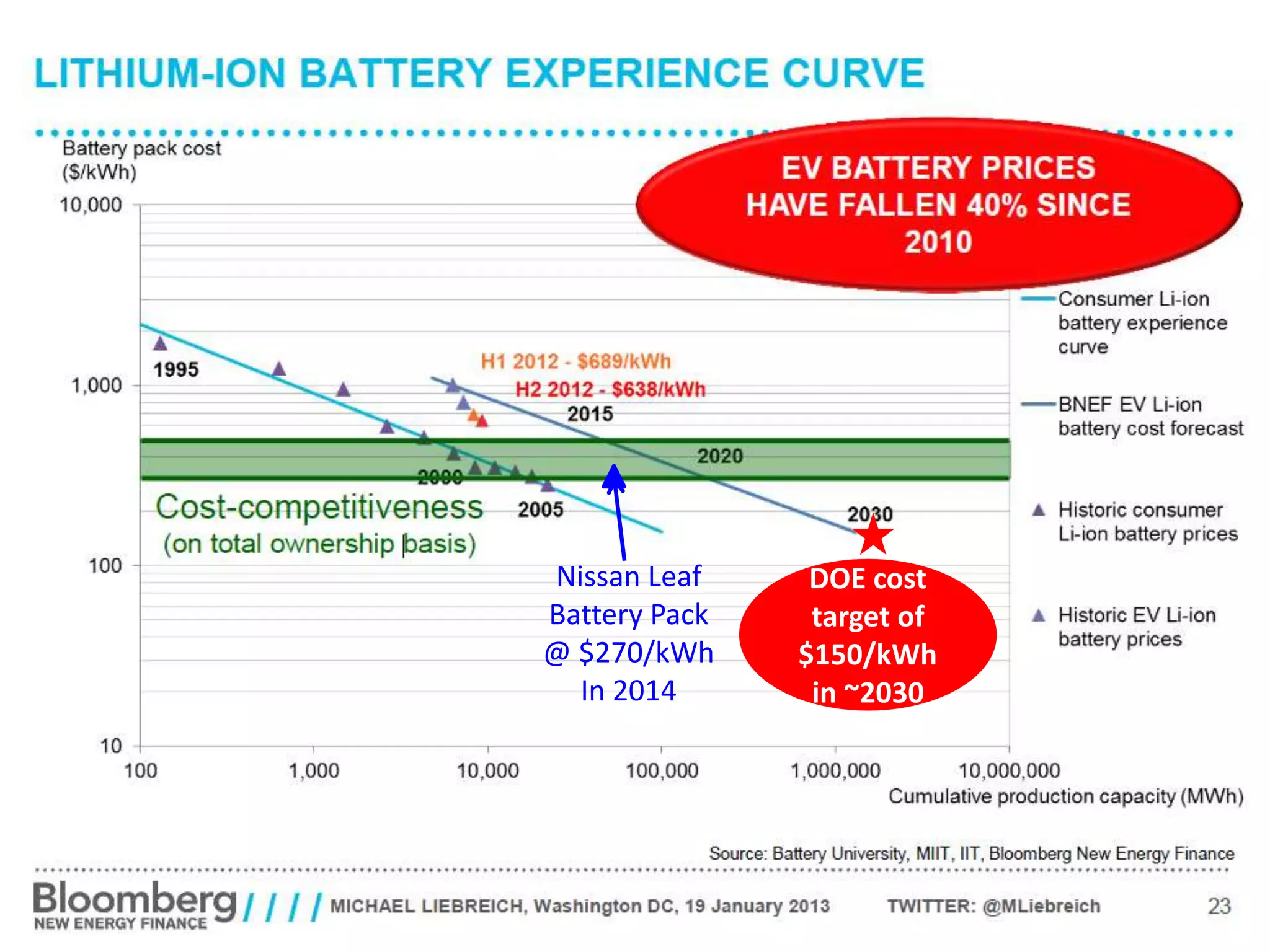
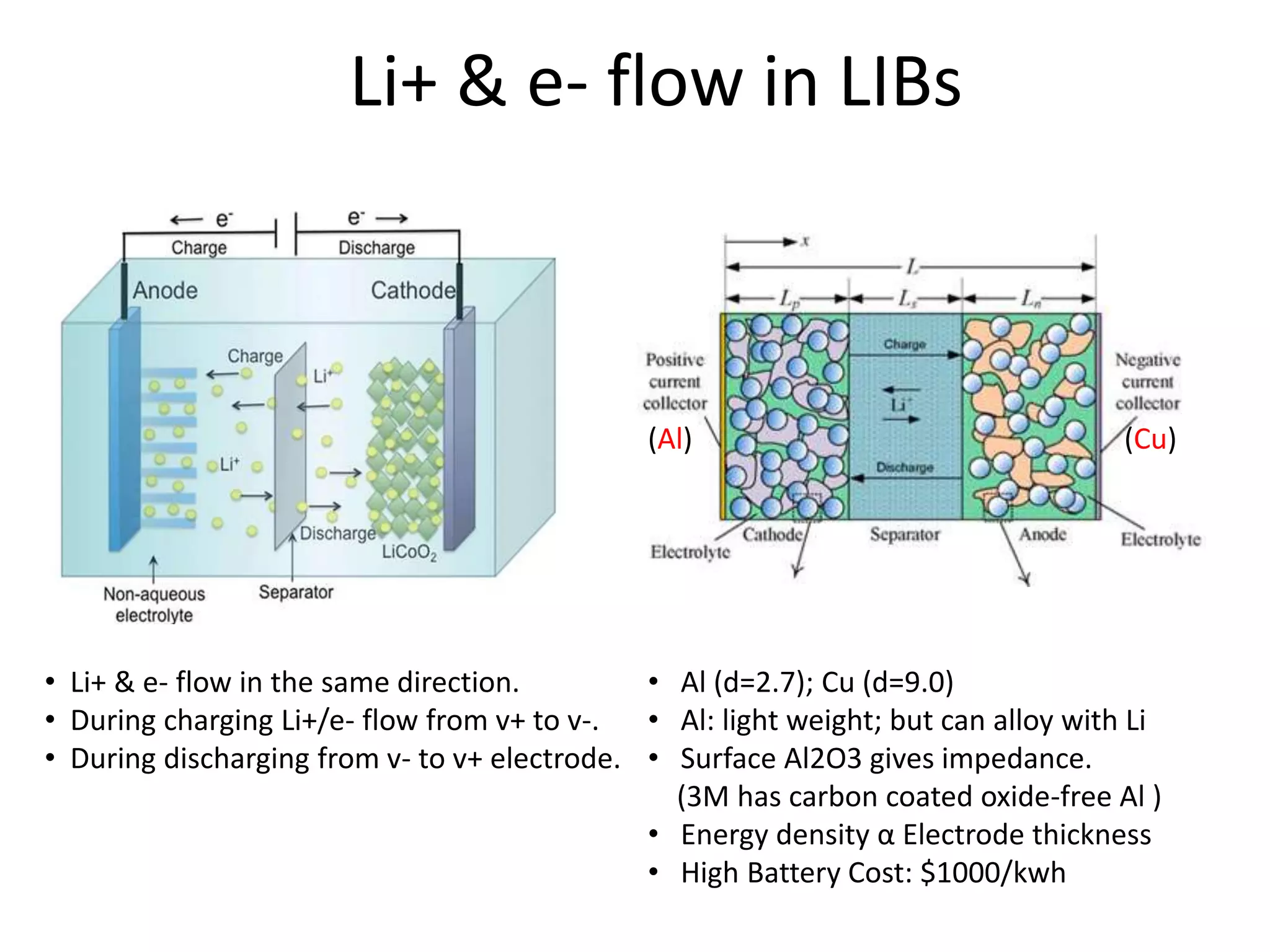
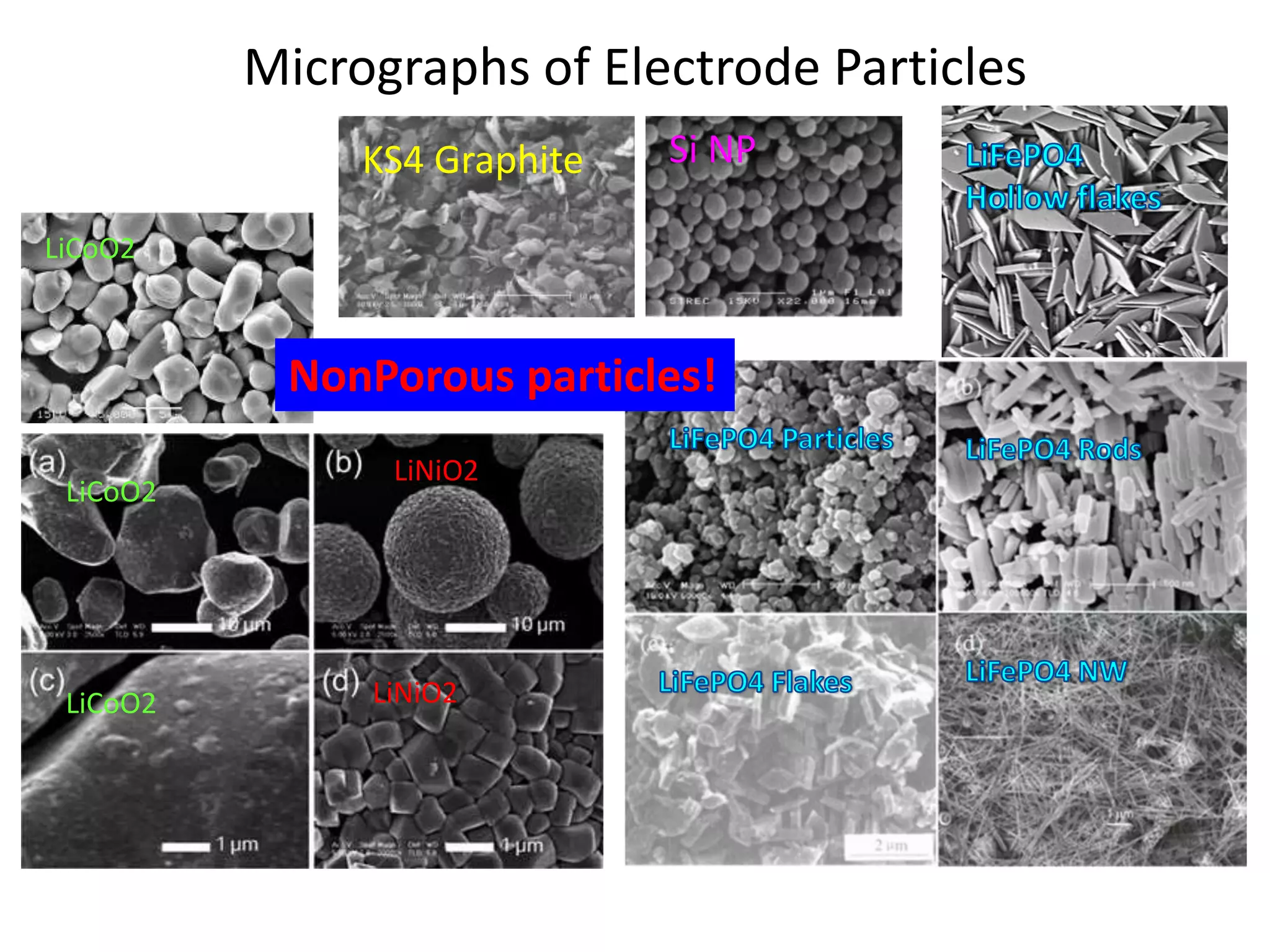
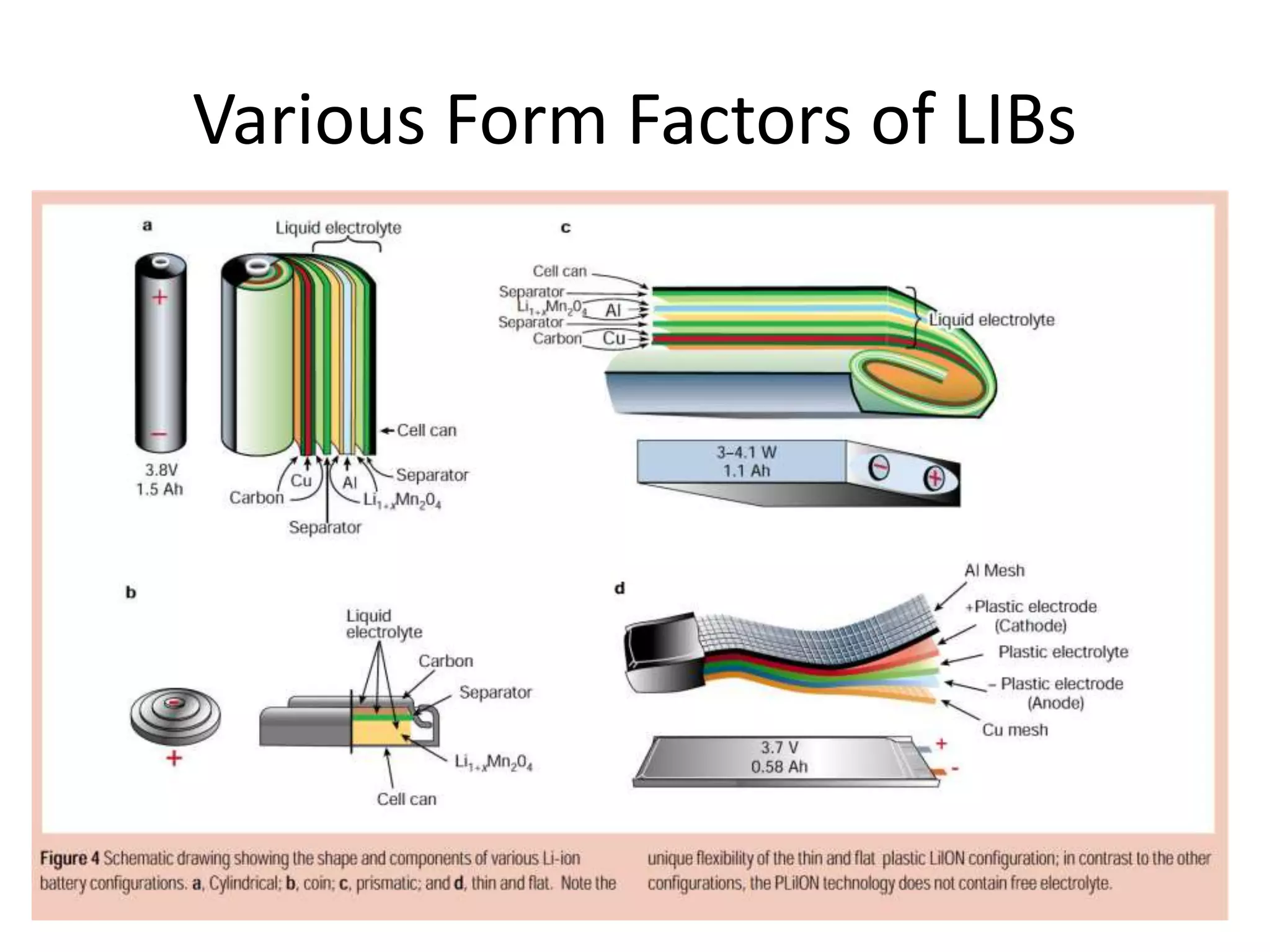
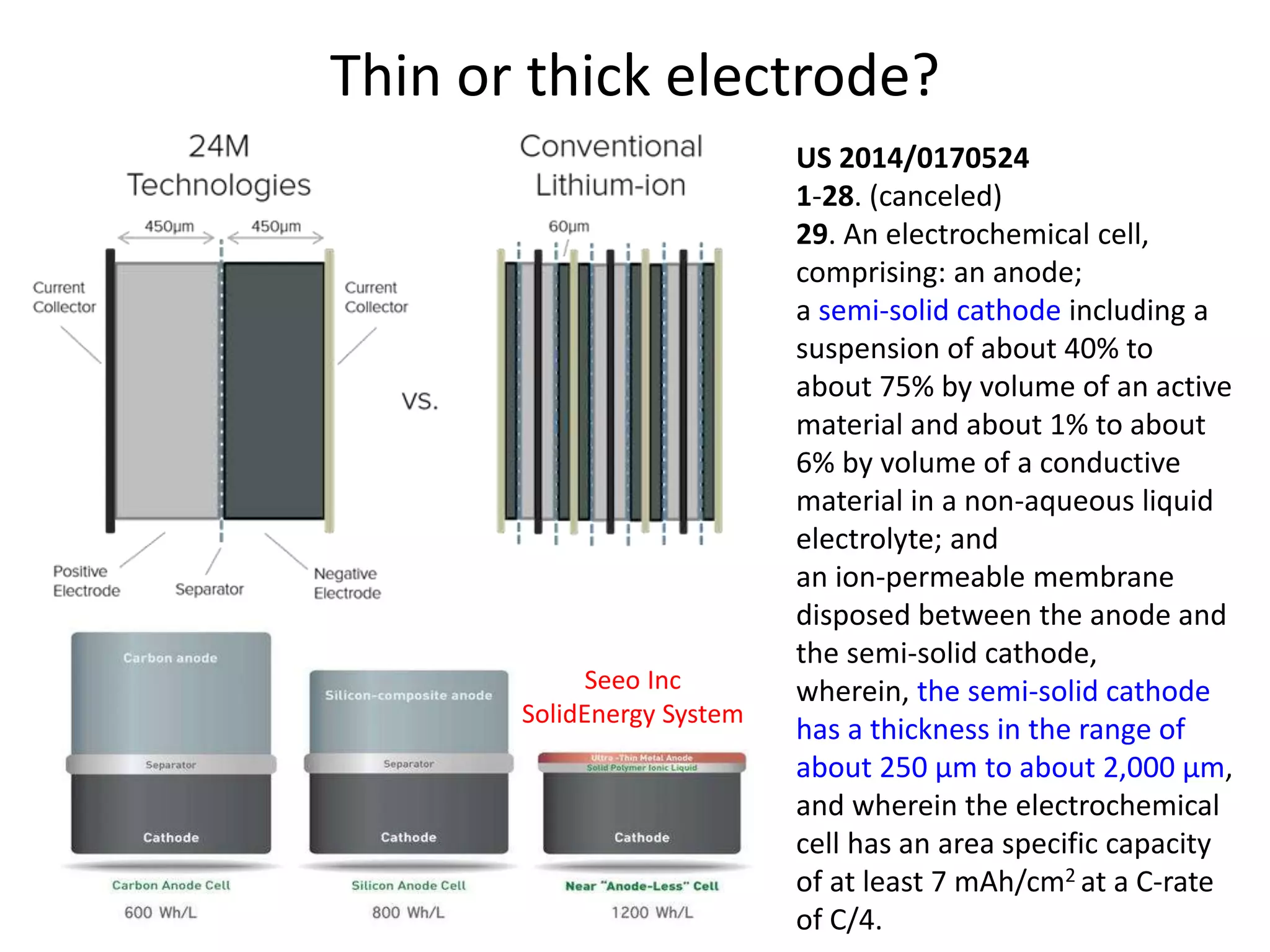
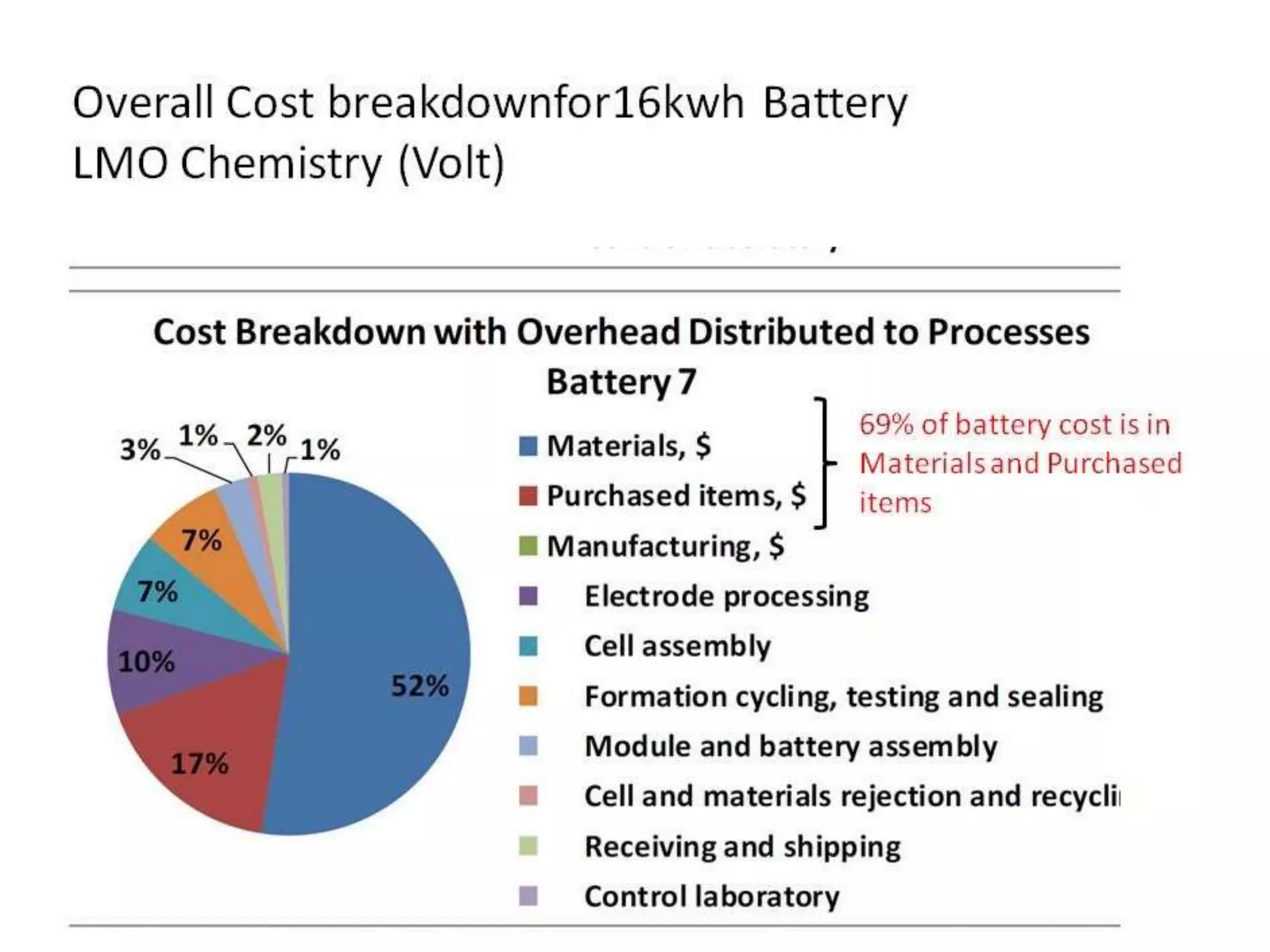
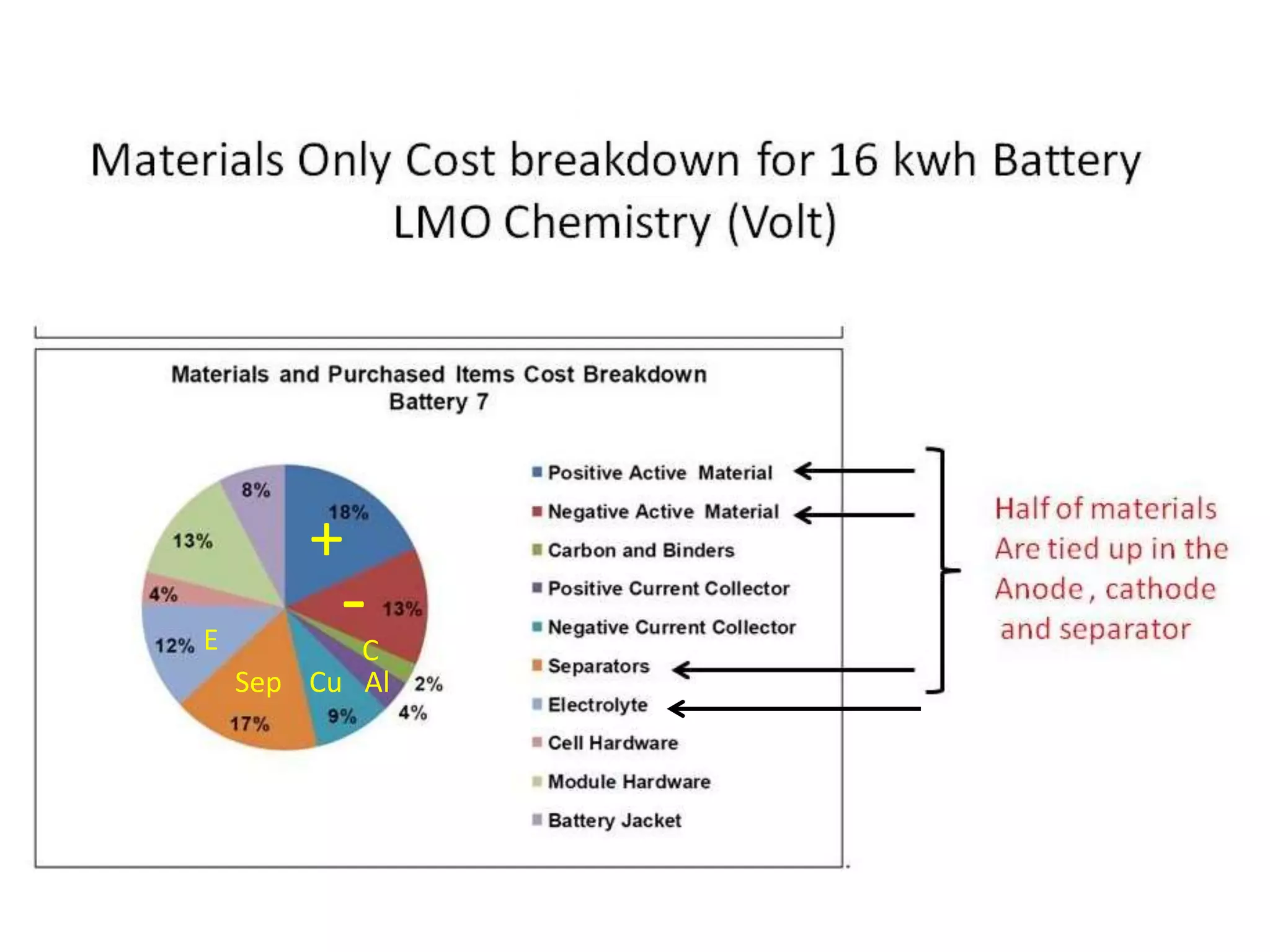
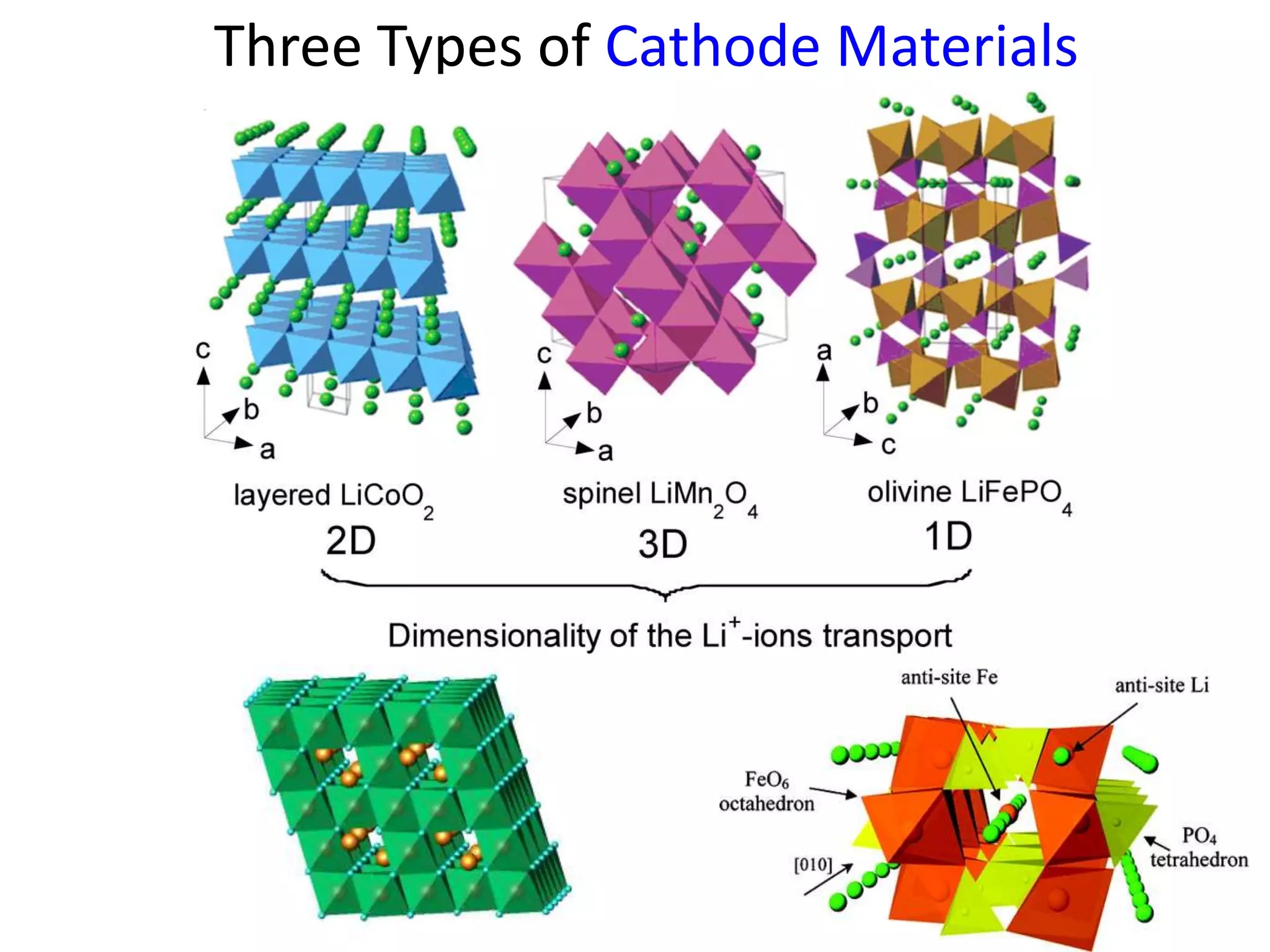
The document provides an overview of lithium-ion battery technologies and opportunities for Taiwan. It discusses that global lithium battery anode materials are highly concentrated in China and Japan, which make up over 95% of the market. It also mentions several US startups working on improved battery materials and technologies. The document examines key areas for improvement in batteries like high voltage cathodes and high capacity anodes. It provides details on various anode and cathode materials being researched. Dendrite suppression methods and the use of coatings, additives, and solid polymer electrolytes are discussed. The opportunities for Taiwan to invest more in energy storage R&D to become a key player are presented.



![List of Important Cathode Materials
voltage specific
capacity
(mAh/g)
energy
density
(wh/kg)
Conductivi
ty
Density
(g/cm3)
[Tapped]
Surface
area
(m2/g)
Cost
($/kg)
LiFePO4 (Fiscar) LFP O 3.4 100-160
(170)
578 E-8 0.23
LiFe1/2Mn1/2PO4 O 3.4-4.1 160
(170)
LiMnPO4 O 4.1 171 701 E-10
LiCoPO4 O 4.8 167 802
LiNiPO4 O 5.1 167 852
LiCoO2 (toxic) (Tesla) LCO L 3.7 120-155
(274 )
570 E-4 25
LiMnO2 L
LiNiO2 (toxic) L 135-180
(274)
13
Li(Ni16/20Co3/20Al1/20)O2 NCA L 3.8
(3-4.2)
180-200
(??)
680-760 4.45 0.5
Li(Ni1/3Mn1/3Co1/3)O2
Nissan & GM
NMC111
BC618
L 4.2-4.6 130-150
(272)
597 4.8
[2.69]
0.26
Li(Ni1/2Mn3/10Co2/10)O2
1/5LiCoO2*4/5(LiMn3/8Ni2/8)O2
NMC532 ??
(164)
635
Li(Ni2/5Mn2/5Co1/5)O2
1/5LiCoO2*4/5(LiMn1/2Ni1/2)O2
NMC442
BC718
??
(155)
4.7
[2.29]
0.39
Li(Ni3/5Mn1/5Co1/5)O2
1/5LiCoO2*2/5(LiMn1/2Ni1/2)O2
NMC622
Li(Ni4/5Mn1/10Co1/10)O2
1/10LiCoO2*4/5(LiMn1/2Ni1/2)O2
NMC811
Lithium Rich Layer Oxide
Li(Li1/3Mn2/3)O2*Li(Mn3/8Ni3/8Co
1/4)O2 = Li(Li, Mn, Ni, Co)O2
HE-NMC
(HE-NCM)
L 4.65
(5.1)
???
(250)
986
LiMn2O4 LMO S 4.3
(3.5-4.3)
100-130
(148)
500
(585)
E-4 4.29 0.5 0.5
LiMn3/2Ni1/2O4
(Nissan, GM)
LMNO
HV-spinel
S 4.7 120
(148)
651 4.45 1.3](https://image.slidesharecdn.com/apragmaticperspectiveonlithiumionbatteries-160123163022/75/A-pragmatic-perspective-on-lithium-ion-batteries-15-2048.jpg)