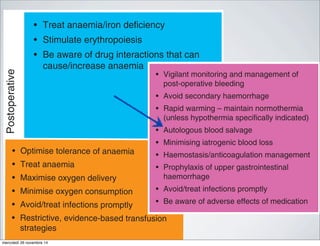
This study analyzed 45 observational studies including over 272,000 patients to determine the association between red blood cell transfusion and morbidity and mortality in high-risk hospitalized patients. The analysis found that in 42 of the 45 studies, the risks of red blood cell transfusion outweighed the benefits, with transfusion associated with increased risk of death, infections, multi-organ dysfunction syndrome, and acute respiratory distress syndrome. A meta-analysis found that transfusion was associated with 70% higher odds of death and 80% higher odds of developing an infectious complication. The study suggests current transfusion practices may need reevaluation given the risks appear to outweigh the benefits in most patients.

![Review Article
Efficacy of red blood cell transfusion in the critically ill:
A systematic review of the literature*
Paul E. Marik, MD, FACP, FCCM, FCCP; Howard L. Corwin, MD, FACP, FCCM, FCCP
In recent years red blood cell
(RBC) transfusion requirements
in western nations has been in-
creasing because of the increasing
burden of chronic disease in an aging
population, improvement in life-support
technology, and blood-intensive surgical
procedures (1, 2). In the United States
alone, nearly 15 million units of blood are
donated and 13 million units are trans-
fused annually (2). For much of the last
(3). On the other hand, it is now becom-
ing clear that there are other important,
less recognized risks of RBC transfusion
related to RBC storage effects and to im-
munomodulating effects of RBC transfu-
sions, which occur in almost all recipi-
ents (4). These immunomodulating*See also p. 2707.
From the Division of Pulmonary and Critical Care
Background: Red blood cell (RBC) transfusions are common in
intensive care unit, trauma, and surgical patients. However, the
hematocrit that should be maintained in any particular patient
because the risks of further transfusion of RBC outweigh the
benefits remains unclear.
Objective: A systematic review of the literature to determine
the association between red blood cell transfusion, and morbidity
and mortality in high-risk hospitalized patients.
Data Sources: MEDLINE, Embase, Cochrane Register of Con-
trolled Trials, and citation review of relevant primary and review
articles.
Study Selection: Cohort studies that assessed the independent
effect of RBC transfusion on patient outcomes. From 571 articles
screened, 45 met inclusion criteria and were included for data
extraction.
Data Extraction: Forty-five studies including 272,596 were
identified (the outcomes from one study were reported in four
separate publications). The outcome measures were mortality,
infections, multiorgan dysfunction syndrome, and acute respira-
tory distress syndrome. The overall risks vs. benefits of RBC
transfusion on patient outcome in each study was classified as (i)
risks outweigh benefits, (ii) neutral risk, and (iii) benefits out-
weigh risks. The odds ratio and 95% confidence interval for each
outcome measure was recorded if available. The pooled odds
ratios were determined using meta-analytic techniques.
Data Synthesis: Forty-five observational studies with a median
of 687 patients/study (range, 63–78,974) were analyzed. In 42 of
the 45 studies the risks of RBC transfusion outweighed the
benefits; the risk was neutral in two studies with the benefits
outweighing the risks in a subgroup of a single study (elderly
patients with an acute myocardial infarction and a hematocrit
<30%). Seventeen of 18 studies, demonstrated that RBC trans-
fusions were an independent predictor of death; the pooled odds
ratio (12 studies) was 1.7 (95% confidence interval, 1.4؊1.9).
Twenty-two studies examined the association between RBC
transfusion and nosocomial infection; in all these studies blood
transfusion was an independent risk factor for infection. The
pooled odds ratio (nine studies) for developing an infectious
complication was 1.8 (95% confidence interval, 1.5–2.2). RBC
transfusions similarly increased the risk of developing multi-
organ dysfunction syndrome (three studies) and acute respiratory
distress syndrome (six studies). The pooled odds ratio for devel-
oping acute respiratory distress syndrome was 2.5 (95% confi-
dence interval, 1.6–3.3).
Conclusions: Despite the inherent limitations in the analysis of
cohort studies, our analysis suggests that in adult, intensive care
unit, trauma, and surgical patients, RBC transfusions are associated
with increased morbidity and mortality and therefore, current trans-
fusion practices may require reevaluation. The risks and benefits of
RBC transfusion should be assessed in every patient before transfu-
sion. (Crit Care Med 2008; 36:2667–2674)
KEY WORDS: blood; blood transfusion; anemia; infections; im-
munomodulation; transfusion-related acute lung injury; acute re-
spiratory distress syndrome; mortality; systematic analysis; meta-
analysis
Morbidity and mortality risk associated with red blood cell
and blood-component transfusion in isolated coronary artery
bypass grafting*
Colleen Gorman Koch, MD, MS; Liang Li, PhD; Andra I. Duncan, MD; Tomislav Mihaljevic, MD;
Delos M. Cosgrove, MD; Floyd D. Loop, MD; Norman J. Starr, MD; Eugene H. Blackstone, MD
A
dministration of packed red
blood cells (PRBCs) has been
associated with morbidity and
mortality for both medical and
surgical patients (1–13). Transfusions are
(2, 8) and long-term mortality (12). Gong
et al. (14) recently demonstrated the as-
sociation between PRBC transfusion and
the development and increased mortality
from acute respiratory distress syndrome.
Our objectives were 1) to exam
whether each unit of PRBC transfu
perioperatively conferred increment
increased risk for mortality and m
morbid outcomes in a large homo
Objective: Our objective was to quantify incremental risk asso-
ciated with transfusion of packed red blood cells and other blood
components on morbidity after coronary artery bypass grafting.
Design: The study design was an observational cohort study.
Setting: This investigation took place at a large tertiary care
referral center.
Patients: A total of 11,963 patients who underwent isolated
coronary artery bypass from January 1, 1995, through July 1,
2002.
Interventions: None.
Measurements and Main Results: Among the 11,963 patients
who underwent isolated coronary artery bypass grafting, 5,814
(48.6%) were transfused. Risk-adjusted probability of developing
in-hospital mortality and morbidity as a function of red blood cell
and blood-component transfusion was modeled using logistic
regression. Transfusion of red blood cells was associated with a
risk-adjusted increased risk for every postoperative morbid ev
mortality (odds ratio [OR], 1.77; 95% confidence interval
1.67–1.87; p < .0001), renal failure (OR, 2.06; 95% CI, 1.87–2
p < .0001), prolonged ventilatory support (OR, 1.79; 95%
1.72–1.86; p < .0001), serious infection (OR, 1.76; 95% CI, 1.68–1
p < .0001), cardiac complications (OR, 1.55; 95% CI, 1.47–1
p < .0001), and neurologic events (OR, 1.37; 95% CI, 1.30–1.44;
.0001).
Conclusions: Perioperative red blood cell transfusion is
single factor most reliably associated with increased risk
postoperative morbid events after isolated coronary artery byp
grafting. Each unit of red cells transfused is associated w
incrementally increased risk for adverse outcome. (Crit Care
2006; 34:1608–1616)
KEY WORDS: blood cells; hemoglobin; complications; cardio
monary bypass; cardiovascular disease; mortality
Transfusion of fresh frozen plasma in critically ill surgical patients
is associated with an increased risk of infection
Babak Sarani, MD, FACS; W. Jonathan Dunkman, BA; Laura Dean; Seema Sonnad, PhD;
Jeffrey I. Rohrbach, RN, MSN; Vicente H. Gracias, MD, FACS
Objective: To determine whether there is an association be-
tween transfusion of fresh frozen plasma and infection in criti-
cally ill surgical patients.
Design: Retrospective study.
Setting: A 24-bed surgical intensive care unit in a university
hospital.
Patients: A total of 380 non-trauma patients who received
fresh frozen plasma from 2004 to 2005 were compared with 2,058
nontrauma patients who did not receive fresh frozen plasma.
Interventions: None.
Measurements and Main Results: We calculated the relative
risk of infectious complication for patients receiving and not
receiving fresh frozen plasma. T-test allowed comparison of av-
erage units of fresh frozen plasma transfused to patients with and
associated pneumonia without shock (relative risk 1.97, 1.03–
3.78), bloodstream infection with shock (relative risk 3.35, 1.69–
6.64), and undifferentiated septic shock (relative risk 3.22, 1.84–
5.61). The relative risk for transfusion of fresh frozen plasma and
all infections was 2.99 (2.28–3.93). The t-test revealed a signifi-
cant dose-response relationship between fresh frozen plasma and
infectious complications (p ؍ .02). Chi-square analysis showed a
significant association between infection and transfusion of fresh
frozen plasma in patients who did not receive concomitant red
blood cell transfusion (p < .01), but this association was not
significant in those who did receive red blood cells in addition to
fresh frozen plasma. The association between fresh frozen
plasma and infectious complications remained significant in the
multivariate model, with an odds ratio of infection per unit of
Allogeneic Blood Transfusion Increases the Risk of
Postoperative Bacterial Infection: A Meta-analysis
Gary E. Hill, MD, William H. Frawley, PhD, Karl E. Griffith, MD, John E. Forestner, MD, and
Joseph P. Minei, MD
Background: Immunosuppression is
a consequence of allogeneic (homologous)
tions that included only the traumatically
injured patient was included in a separate
subgroup of trauma patien
(range, 5.03–5.43), with all stud
The Journal of TRAUMA Injury, Infection, and C
mercoledì 26 novembre 14](https://image.slidesharecdn.com/romaint-160320122837/85/A-fresh-look-at-cell-salvage-3-320.jpg)











![FORMAZIONE
USA
• PBMT : Perioperative Blood Management
Technologist
ESAME
K = conoscenza
S = abilità
A = pratica
Perioperative Blood Management Technologist [PBMT]
Job Domain Analysis
Theoretical Hierarchical Construct for K/S/A for Competency Exam
Respond correctly to
critical incidents and
emergencies [4.3]
Follow guideline
indications for use and
record keeping [3.3]
Disposable supplies
and interface with
hardware [2.3]
Inter-team member
communication and
patient privacy [1.3}
Communication with
team during critical
incident and crisis
management [4.4]
Follow guidelines
recognizing
contraindications and
exceptions [3.4]
Follow manufacturer
instructions-for-use
and assembly [2.4]
Integration into surgical
team and participate in
care planning and quality
management [1.4]
Design and practice
team drills for critical
incidents [4.5]
Suggest changes to and
author clinical procedure
guidelines [3.5]
Application and
operation of
equipment [2.5]
Assertiveness, lead team
when required [1.5]
Critical
Incidents
Patient Care
Procedures
Equipment /
Disposables
Environmental
Factors
K/S/A Label Count Percent
K Knowledge 45 0.41
S Skills 31 0.28
A Application 34 0.31
Total 110 1.00
mercoledì 26 novembre 14](https://image.slidesharecdn.com/romaint-160320122837/85/A-fresh-look-at-cell-salvage-19-320.jpg)











![reinfusion of salvaged blood was continued without the LDF and no hypotension occurred. This is a
recognised complication which may be related to elevated levels of interleukin 6 [71], and is reviewed
by Sreelakshmi [72].
Learning points
The use of leucodepletion filters (LDF) with cell salvaged blood can, rarely, cause significant
hypotension
Stopping the infusion and resuscitation with fluids and vasopressors may be necessary although
all reports describe only transient hypotension
In cases where there is brisk haemorrhage and the blood is needed, try infusing without the LDF
Recommendations
Ensure that all cell salvage users in your institution are made aware of this complication and the
simple measures that need to be taken should it occur
Action: Hospital Transfusion Committees (HTC), Hospital Transfusion Teams (HTT)
Ensure all cases of serious reactions are reported to SHOT via the hospital transfusion team
Action: HTTs, Operating Department Practitioners, Cell Salvage Operators
Consider where a machine failure occurs, which is not due to operator error, these are reported
to the Medicines and Healthcare products Regulatory Agency (MHRA) under the Medical Devices
reporting schememercoledì 26 novembre 14](https://image.slidesharecdn.com/romaint-160320122837/85/A-fresh-look-at-cell-salvage-37-320.jpg)


![plasma, and cryoprecipitate. Anticipate coagulation factor
deficiency after more than 2 litres blood loss with continued bleed-
ing and repeat full blood count, prothrombin time, and activated
partial thromboplastin time and fibrinogen levels after the reinfu-
sion of each litre of salvaged blood in order to detect and appropri-
ately treat coagulapathy (Table 1).
General indications for cell salvage
(i) Anticipated intraoperative blood loss .1 litre or .20% of
blood volume.
(ii) Preoperative anaemia or increased risk factors for bleeding.
(iii) Patients with rare blood group or antibodies.
(iv) Patient refusal to receive allogeneic blood transfusion.
(v) The American Association of Blood Banks suggest cell
salvage is indicated in surgery where blood would ordinarily
be cross-matched or where more than 10% of patients under-
going the procedure require transfusion.
allo
fixe
requ
pro
was
cran
plas
Sp
Cel
enc
ord
in p
pro
afte
Hom
safe
Perioperative cell salvage
Lakshminarasimhan Kuppurao MD DA DNB FRCA
Michael Wee BSc (Hons) MBChB FRCA
The National Blood Service for England col-
lects, tests, processes, stores, and issues 2.1
million blood donations each year, and the
optimal use of this scarce resource is of para-
mount importance. Allogeneic red blood cell
(RBC) transfusion is associated with well-
known adverse effects. These include febrile,
anaphylactic, and haemolytic transfusion reac-
Key points
Complications of allogeneic
transfusion are rare but can
be life threatening.
There is a drive to reduce
allogeneic blood transfusion
due to cost and scarcity.
Cell salvage should be used
e cell salvage
purao MD DA DNB FRCA
s) MBChB FRCA
The National Blood Service for England col-
lects, tests, processes, stores, and issues 2.1
million blood donations each year, and the
optimal use of this scarce resource is of para-
mount importance. Allogeneic red blood cell
(RBC) transfusion is associated with well-
known adverse effects. These include febrile,
anaphylactic, and haemolytic transfusion reac-
tions, transfusion-related acute lung injury, and
transfusion-associated circulatory overload. In
addition, although rare, there are infection risks
of viral, bacterial, parasitic, or prion trans-
mission. In the laboratory setting, allogeneic
involves filtering and washing to remove con-
taminants. Red cells are retained, while the
plasma, platelets, heparin, free haemoglobin,
and inflammatory mediators are discarded with
the wash solution. This process may be discon-
tinuous or continuous, and the resulting red
cells are finally resuspended in normal saline at
a haematocrit of 50–70%, and reinfused into
the patient. Once primed, the cell salvage
machine should be used within 8 h to prevent
infective complications.
Benefits of cell salvage
Matrix reference 1A06
evolved since its inception in the 1960s.
Initially, cell salvage was limited to simply fil-
tering blood loss during surgery by gravity.
More modern devices collect blood to which is
added heparinized normal saline or citrate
anticoagulant. Processing the collected blood
activation of intravascular coagulation
increased capillary permeability causing
lung injury and renal failure. This syndr
related to the dilution of salvaged blood
large quantities of saline solution,
creates deposits of cellular aggregates
doi:10.1093/bjaceaccp/mkq017 Advance Access publication 26 M
Continuing Education in Anaesthesia, Critical Care & Pain | Volume 10 Number 4 2010
& The Author [2010]. Published by Oxford University Press on behalf of the British Journal of Anaesthesia.
All rights reserved. For Permissions, please email: journals.permissions@oxfordjournal.org
mercoledì 26 novembre 14](https://image.slidesharecdn.com/romaint-160320122837/85/A-fresh-look-at-cell-salvage-41-320.jpg)











![Intraoperative blood salvage in cancer surgery:
safe and effective?
Ernil Hansen *, Volker Bechmann, Juergen Altmeppen
Department of Anesthesiologie, University of Regensburg, D-93042 Regensburg, Germany
Abstract
To support blood supply in the growing field of cancer surgery and to avoid transfusion induced immunomodulation
caused by the allogeneic barrier and by blood storage leasions we use intraoperative blood salvage with blood irra-
diation. This method is safe as it provides efficient elimination of contaminating cancer cells, and as it does not
compromise the quality of RBC. According to our experience with more than 700 procedures the combination of blood
salvage with blood irradiation also is very effective in saving blood resources. With this autologous, fresh, washed RBC
a blood product of excellent quality is available for optimal hemotherapy in cancer patients.
Ó 2002 Elsevier Science Ltd. All rights reserved.
1. Introduction
The demand for blood in cancer surgery is high
and increasing. Problems with the supply of com-
patible blood are not uncommon in these patients
that previously have seen surgery and transfusions.
Some transfusion risks are especially relevant to
cancer patients like immunomodulation with im-
donations suffers from the poor predictability of
intraoperative blood loss leading to a waste of
autologous blood, or to insufficient supply. Im-
munosuppression is not only caused by the allog-
eneic barrier, but also by cell lesions during blood
storage at low temperature [2], relevant to both
allogeneic and autologous banked blood. In ad-
dition, growth factors are released during storage
www.elsevier.com/locate/transci
Intraoperative blood salvage in cancer surgery
safe and effective?
Ernil Hansen *, Volker Bechmann, Juergen Altmeppen
Department of Anesthesiologie, University of Regensburg, D-93042 Regensburg, Germany
act
support blood supply in the growing field of cancer surgery and to avoid transfusion induced imm
d by the allogeneic barrier and by blood storage leasions we use intraoperative blood salvage w
www.elsevier.
Transfusion and Apheresis Science 27 (2002) 153–157
Fig. 1. Transfusion risks most relevant to cancer patients.
E. Hansen et al. / Transfusion and Apheresis Science 27 (2002) 153–157
più di 700 casi
irradiazione GRC
50Gy
diminuzione cellule
tumorali Log 12
ottima qualità,
sopravvivenza,
funzione
mercoledì 26 novembre 14](https://image.slidesharecdn.com/romaint-160320122837/85/A-fresh-look-at-cell-salvage-53-320.jpg)
![2011 Update to The Society of Thoracic Surgeons
and the Society of Cardiovascular Anesthesiologists
Blood Conservation Clinical Practice Guidelines*
The Society of Thoracic Surgeons Blood Conservation Guideline Task Force:
Victor A. Ferraris, MD, PhD (Chair), Jeremiah R. Brown, PhD, George J. Despotis, MD,
John W. Hammon, MD, T. Brett Reece, MD, Sibu P. Saha, MD, MBA,
Howard K. Song, MD, PhD, and Ellen R. Clough, PhD
The Society of Cardiovascular Anesthesiologists Special Task Force on Blood Transfusion:
Linda J. Shore-Lesserson, MD, Lawrence T. Goodnough, MD, C. David Mazer, MD,
Aryeh Shander, MD, Mark Stafford-Smith, MD, and Jonathan Waters, MD
The International Consortium for Evidence Based Perfusion:
Robert A. Baker, PhD, Dip Perf, CCP (Aus), Timothy A. Dickinson, MS,
Daniel J. FitzGerald, CCP, LP, Donald S. Likosky, PhD, and Kenneth G. Shann, CCP
Division of Cardiovascular and Thoracic Surgery, University of Kentucky, Lexington, Kentucky (VAF, SPS), Department of
Anesthesiology, University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania (JW), Departments of Anesthesiology and Critical
Care Medicine, Englewood Hospital and Medical Center, Englewood, New Jersey (AS), Departments of Pathology and Medicine,
Stanford University School of Medicine, Stanford, California (LTG), Departments of Anesthesiology and Cardiothoracic Surgery,
Montefiore Medical Center, Bronx, New York (LJS-L, KGS), Departments of Anesthesiology, Immunology, and Pathology, Washington
University School of Medicine, St. Louis, Missouri (GJD), Dartmouth Institute for Health Policy and Clinical Practice, Section of
Cardiology, Dartmouth Medical School, Lebanon, New Hampshire (JRB), Department of Cardiothoracic Surgery, Wake Forest School of
Medicine, Winston-Salem, North Carolina (JWH), Department of Anesthesia, St. Michael’s Hospital, University of Toronto, Toronto,
Ontario (CDM), Cardiac Surgical Research Group, Flinders Medical Centre, South Australia, Australia (RAB), Department of Surgery,
Medicine, Community and Family Medicine, and the Dartmouth Institute for Health Policy and Clinical Practice, Dartmouth Medical
School, Hanover, New Hampshire (DSL), SpecialtyCare, Nashville, Tennessee (TAD), Department of Cardiac Surgery, Brigham and
Women’s Hospital, Harvard University, Boston, Massachusetts (DJF), Division of Cardiothoracic Surgery, Oregon Health and Science
University Medical Center, Portland, Oregon (HKS), Department of Cardiothoracic Surgery, University of Colorado Health Sciences
Center, Aurora, Colorado (TBR), Department of Anesthesiology, Duke University Medical Center, Durham, North Carolina (MS-S), and
The Society of Thoracic Surgeons, Chicago, Illinois (ERC)
Background. Practice guidelines reflect published liter- Methods. The search methods used in the current
pro-
bolic
ports
with
213].
t re-
lica-
that
om-
g, or
per-
ICU
Two
ship
pa-
volv-
diac
tar-
mbo-
able [227], and addition of factor concentrates augments
multiple other interventions. Fractionated factor concen-
trates, like factor IX concentrates or one of its various
forms (Beriplex or factor VIII inhibitor bypassing activ-
ity), are considered “secondary components” and may be
acceptable to some Jehovah’s Witness patients [222].
Addition of factor IX concentrates may be most useful in
the highest risk Jehovah’s Witness patients.
d) Blood Salvage Interventions
EXPANDED USE OF RED CELL SALVAGE USING CENTRIFUGATION
Class IIb.
1. In high-risk patients with known malignancy who
require CPB, blood salvage using centrifugation of
blood salvaged from the operative field may be
considered since substantial data support benefit in
patients without malignancy, and new evidence
suggests worsened outcome when allogeneic trans-
fusion is required in patients with malignancy.
(Level of evidence B)
In 1986, the American Medical Association Council on
Scientific Affairs issued a statement regarding the safety
of blood salvage during cancer surgery [228]. At that
time, they advised against its use. Since then, 10 obser-
vational studies that included 476 patients who received
blood salvage during resection of multiple different tumor
types involving the liver [229–231], prostate [232–234],
uterus [235, 236], and urologic system [237, 238] support the
use of salvage of red cells using centrifugation in cancer
patients. In seven studies, a control group received no
transfusion, allogeneic transfusion, or preoperative autolo-
end of CPB is reasonable as part of a bl
agement program to minimize blood tr
(Level of evidence C)
2. Centrifugation instead of direct infusion o
pump blood is reasonable for minimizing
allogeneic RBC transfusion. (Level of evi
Most surgical teams reinfuse blood from t
poreal circuit (ECC) back into patients at the
as part of a blood conservation strategy. Cu
blood salvaging techniques exist: (1) direct
post-CPB circuit blood with no processing;
cessing of the circuit blood, either by centrifu
ultrafiltration, to remove either plasma com
water soluble components from blood before
Ann Thorac Surg FERRARIS
2011;91:944–82 STS BLOOD CONSERVATION REVISION
mercoledì 26 novembre 14](https://image.slidesharecdn.com/romaint-160320122837/85/A-fresh-look-at-cell-salvage-54-320.jpg)
![2011 Update to The Society of Thoracic Surgeons
and the Society of Cardiovascular Anesthesiologists
Blood Conservation Clinical Practice Guidelines*
The Society of Thoracic Surgeons Blood Conservation Guideline Task Force:
Victor A. Ferraris, MD, PhD (Chair), Jeremiah R. Brown, PhD, George J. Despotis, MD,
John W. Hammon, MD, T. Brett Reece, MD, Sibu P. Saha, MD, MBA,
Howard K. Song, MD, PhD, and Ellen R. Clough, PhD
The Society of Cardiovascular Anesthesiologists Special Task Force on Blood Transfusion:
Linda J. Shore-Lesserson, MD, Lawrence T. Goodnough, MD, C. David Mazer, MD,
Aryeh Shander, MD, Mark Stafford-Smith, MD, and Jonathan Waters, MD
The International Consortium for Evidence Based Perfusion:
Robert A. Baker, PhD, Dip Perf, CCP (Aus), Timothy A. Dickinson, MS,
Daniel J. FitzGerald, CCP, LP, Donald S. Likosky, PhD, and Kenneth G. Shann, CCP
Division of Cardiovascular and Thoracic Surgery, University of Kentucky, Lexington, Kentucky (VAF, SPS), Department of
Anesthesiology, University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania (JW), Departments of Anesthesiology and Critical
Care Medicine, Englewood Hospital and Medical Center, Englewood, New Jersey (AS), Departments of Pathology and Medicine,
Stanford University School of Medicine, Stanford, California (LTG), Departments of Anesthesiology and Cardiothoracic Surgery,
Montefiore Medical Center, Bronx, New York (LJS-L, KGS), Departments of Anesthesiology, Immunology, and Pathology, Washington
University School of Medicine, St. Louis, Missouri (GJD), Dartmouth Institute for Health Policy and Clinical Practice, Section of
Cardiology, Dartmouth Medical School, Lebanon, New Hampshire (JRB), Department of Cardiothoracic Surgery, Wake Forest School of
Medicine, Winston-Salem, North Carolina (JWH), Department of Anesthesia, St. Michael’s Hospital, University of Toronto, Toronto,
Ontario (CDM), Cardiac Surgical Research Group, Flinders Medical Centre, South Australia, Australia (RAB), Department of Surgery,
Medicine, Community and Family Medicine, and the Dartmouth Institute for Health Policy and Clinical Practice, Dartmouth Medical
School, Hanover, New Hampshire (DSL), SpecialtyCare, Nashville, Tennessee (TAD), Department of Cardiac Surgery, Brigham and
Women’s Hospital, Harvard University, Boston, Massachusetts (DJF), Division of Cardiothoracic Surgery, Oregon Health and Science
University Medical Center, Portland, Oregon (HKS), Department of Cardiothoracic Surgery, University of Colorado Health Sciences
Center, Aurora, Colorado (TBR), Department of Anesthesiology, Duke University Medical Center, Durham, North Carolina (MS-S), and
The Society of Thoracic Surgeons, Chicago, Illinois (ERC)
Background. Practice guidelines reflect published liter- Methods. The search methods used in the current
pro-
bolic
ports
with
213].
t re-
lica-
that
om-
g, or
per-
ICU
Two
ship
pa-
volv-
diac
tar-
mbo-
able [227], and addition of factor concentrates augments
multiple other interventions. Fractionated factor concen-
trates, like factor IX concentrates or one of its various
forms (Beriplex or factor VIII inhibitor bypassing activ-
ity), are considered “secondary components” and may be
acceptable to some Jehovah’s Witness patients [222].
Addition of factor IX concentrates may be most useful in
the highest risk Jehovah’s Witness patients.
d) Blood Salvage Interventions
EXPANDED USE OF RED CELL SALVAGE USING CENTRIFUGATION
Class IIb.
1. In high-risk patients with known malignancy who
require CPB, blood salvage using centrifugation of
blood salvaged from the operative field may be
considered since substantial data support benefit in
patients without malignancy, and new evidence
suggests worsened outcome when allogeneic trans-
fusion is required in patients with malignancy.
(Level of evidence B)
In 1986, the American Medical Association Council on
Scientific Affairs issued a statement regarding the safety
of blood salvage during cancer surgery [228]. At that
time, they advised against its use. Since then, 10 obser-
vational studies that included 476 patients who received
blood salvage during resection of multiple different tumor
types involving the liver [229–231], prostate [232–234],
uterus [235, 236], and urologic system [237, 238] support the
use of salvage of red cells using centrifugation in cancer
patients. In seven studies, a control group received no
transfusion, allogeneic transfusion, or preoperative autolo-
end of CPB is reasonable as part of a bl
agement program to minimize blood tr
(Level of evidence C)
2. Centrifugation instead of direct infusion o
pump blood is reasonable for minimizing
allogeneic RBC transfusion. (Level of evi
Most surgical teams reinfuse blood from t
poreal circuit (ECC) back into patients at the
as part of a blood conservation strategy. Cu
blood salvaging techniques exist: (1) direct
post-CPB circuit blood with no processing;
cessing of the circuit blood, either by centrifu
ultrafiltration, to remove either plasma com
water soluble components from blood before
Ann Thorac Surg FERRARIS
2011;91:944–82 STS BLOOD CONSERVATION REVISION
10 studi osservazionali su 476 pazienti
operati per diverse patologie tumorali
supportano l’uso del cell saver
mercoledì 26 novembre 14](https://image.slidesharecdn.com/romaint-160320122837/85/A-fresh-look-at-cell-salvage-55-320.jpg)
![2011 Update to The Society of Thoracic Surgeons
and the Society of Cardiovascular Anesthesiologists
Blood Conservation Clinical Practice Guidelines*
The Society of Thoracic Surgeons Blood Conservation Guideline Task Force:
Victor A. Ferraris, MD, PhD (Chair), Jeremiah R. Brown, PhD, George J. Despotis, MD,
John W. Hammon, MD, T. Brett Reece, MD, Sibu P. Saha, MD, MBA,
Howard K. Song, MD, PhD, and Ellen R. Clough, PhD
The Society of Cardiovascular Anesthesiologists Special Task Force on Blood Transfusion:
Linda J. Shore-Lesserson, MD, Lawrence T. Goodnough, MD, C. David Mazer, MD,
Aryeh Shander, MD, Mark Stafford-Smith, MD, and Jonathan Waters, MD
The International Consortium for Evidence Based Perfusion:
Robert A. Baker, PhD, Dip Perf, CCP (Aus), Timothy A. Dickinson, MS,
Daniel J. FitzGerald, CCP, LP, Donald S. Likosky, PhD, and Kenneth G. Shann, CCP
Division of Cardiovascular and Thoracic Surgery, University of Kentucky, Lexington, Kentucky (VAF, SPS), Department of
Anesthesiology, University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania (JW), Departments of Anesthesiology and Critical
Care Medicine, Englewood Hospital and Medical Center, Englewood, New Jersey (AS), Departments of Pathology and Medicine,
Stanford University School of Medicine, Stanford, California (LTG), Departments of Anesthesiology and Cardiothoracic Surgery,
Montefiore Medical Center, Bronx, New York (LJS-L, KGS), Departments of Anesthesiology, Immunology, and Pathology, Washington
University School of Medicine, St. Louis, Missouri (GJD), Dartmouth Institute for Health Policy and Clinical Practice, Section of
Cardiology, Dartmouth Medical School, Lebanon, New Hampshire (JRB), Department of Cardiothoracic Surgery, Wake Forest School of
Medicine, Winston-Salem, North Carolina (JWH), Department of Anesthesia, St. Michael’s Hospital, University of Toronto, Toronto,
Ontario (CDM), Cardiac Surgical Research Group, Flinders Medical Centre, South Australia, Australia (RAB), Department of Surgery,
Medicine, Community and Family Medicine, and the Dartmouth Institute for Health Policy and Clinical Practice, Dartmouth Medical
School, Hanover, New Hampshire (DSL), SpecialtyCare, Nashville, Tennessee (TAD), Department of Cardiac Surgery, Brigham and
Women’s Hospital, Harvard University, Boston, Massachusetts (DJF), Division of Cardiothoracic Surgery, Oregon Health and Science
University Medical Center, Portland, Oregon (HKS), Department of Cardiothoracic Surgery, University of Colorado Health Sciences
Center, Aurora, Colorado (TBR), Department of Anesthesiology, Duke University Medical Center, Durham, North Carolina (MS-S), and
The Society of Thoracic Surgeons, Chicago, Illinois (ERC)
Background. Practice guidelines reflect published liter- Methods. The search methods used in the current
pro-
bolic
ports
with
213].
t re-
lica-
that
om-
g, or
per-
ICU
Two
ship
pa-
volv-
diac
tar-
mbo-
able [227], and addition of factor concentrates augments
multiple other interventions. Fractionated factor concen-
trates, like factor IX concentrates or one of its various
forms (Beriplex or factor VIII inhibitor bypassing activ-
ity), are considered “secondary components” and may be
acceptable to some Jehovah’s Witness patients [222].
Addition of factor IX concentrates may be most useful in
the highest risk Jehovah’s Witness patients.
d) Blood Salvage Interventions
EXPANDED USE OF RED CELL SALVAGE USING CENTRIFUGATION
Class IIb.
1. In high-risk patients with known malignancy who
require CPB, blood salvage using centrifugation of
blood salvaged from the operative field may be
considered since substantial data support benefit in
patients without malignancy, and new evidence
suggests worsened outcome when allogeneic trans-
fusion is required in patients with malignancy.
(Level of evidence B)
In 1986, the American Medical Association Council on
Scientific Affairs issued a statement regarding the safety
of blood salvage during cancer surgery [228]. At that
time, they advised against its use. Since then, 10 obser-
vational studies that included 476 patients who received
blood salvage during resection of multiple different tumor
types involving the liver [229–231], prostate [232–234],
uterus [235, 236], and urologic system [237, 238] support the
use of salvage of red cells using centrifugation in cancer
patients. In seven studies, a control group received no
transfusion, allogeneic transfusion, or preoperative autolo-
end of CPB is reasonable as part of a bl
agement program to minimize blood tr
(Level of evidence C)
2. Centrifugation instead of direct infusion o
pump blood is reasonable for minimizing
allogeneic RBC transfusion. (Level of evi
Most surgical teams reinfuse blood from t
poreal circuit (ECC) back into patients at the
as part of a blood conservation strategy. Cu
blood salvaging techniques exist: (1) direct
post-CPB circuit blood with no processing;
cessing of the circuit blood, either by centrifu
ultrafiltration, to remove either plasma com
water soluble components from blood before
Ann Thorac Surg FERRARIS
2011;91:944–82 STS BLOOD CONSERVATION REVISION
10 studi osservazionali su 476 pazienti
operati per diverse patologie tumorali
supportano l’uso del cell saver
mercoledì 26 novembre 14](https://image.slidesharecdn.com/romaint-160320122837/85/A-fresh-look-at-cell-salvage-56-320.jpg)
![2011 Update to The Society of Thoracic Surgeons
and the Society of Cardiovascular Anesthesiologists
Blood Conservation Clinical Practice Guidelines*
The Society of Thoracic Surgeons Blood Conservation Guideline Task Force:
Victor A. Ferraris, MD, PhD (Chair), Jeremiah R. Brown, PhD, George J. Despotis, MD,
John W. Hammon, MD, T. Brett Reece, MD, Sibu P. Saha, MD, MBA,
Howard K. Song, MD, PhD, and Ellen R. Clough, PhD
The Society of Cardiovascular Anesthesiologists Special Task Force on Blood Transfusion:
Linda J. Shore-Lesserson, MD, Lawrence T. Goodnough, MD, C. David Mazer, MD,
Aryeh Shander, MD, Mark Stafford-Smith, MD, and Jonathan Waters, MD
The International Consortium for Evidence Based Perfusion:
Robert A. Baker, PhD, Dip Perf, CCP (Aus), Timothy A. Dickinson, MS,
Daniel J. FitzGerald, CCP, LP, Donald S. Likosky, PhD, and Kenneth G. Shann, CCP
Division of Cardiovascular and Thoracic Surgery, University of Kentucky, Lexington, Kentucky (VAF, SPS), Department of
Anesthesiology, University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania (JW), Departments of Anesthesiology and Critical
Care Medicine, Englewood Hospital and Medical Center, Englewood, New Jersey (AS), Departments of Pathology and Medicine,
Stanford University School of Medicine, Stanford, California (LTG), Departments of Anesthesiology and Cardiothoracic Surgery,
Montefiore Medical Center, Bronx, New York (LJS-L, KGS), Departments of Anesthesiology, Immunology, and Pathology, Washington
University School of Medicine, St. Louis, Missouri (GJD), Dartmouth Institute for Health Policy and Clinical Practice, Section of
Cardiology, Dartmouth Medical School, Lebanon, New Hampshire (JRB), Department of Cardiothoracic Surgery, Wake Forest School of
Medicine, Winston-Salem, North Carolina (JWH), Department of Anesthesia, St. Michael’s Hospital, University of Toronto, Toronto,
Ontario (CDM), Cardiac Surgical Research Group, Flinders Medical Centre, South Australia, Australia (RAB), Department of Surgery,
Medicine, Community and Family Medicine, and the Dartmouth Institute for Health Policy and Clinical Practice, Dartmouth Medical
School, Hanover, New Hampshire (DSL), SpecialtyCare, Nashville, Tennessee (TAD), Department of Cardiac Surgery, Brigham and
Women’s Hospital, Harvard University, Boston, Massachusetts (DJF), Division of Cardiothoracic Surgery, Oregon Health and Science
University Medical Center, Portland, Oregon (HKS), Department of Cardiothoracic Surgery, University of Colorado Health Sciences
Center, Aurora, Colorado (TBR), Department of Anesthesiology, Duke University Medical Center, Durham, North Carolina (MS-S), and
The Society of Thoracic Surgeons, Chicago, Illinois (ERC)
Background. Practice guidelines reflect published liter- Methods. The search methods used in the current
pro-
bolic
ports
with
213].
t re-
lica-
that
om-
g, or
per-
ICU
Two
ship
pa-
volv-
diac
tar-
mbo-
able [227], and addition of factor concentrates augments
multiple other interventions. Fractionated factor concen-
trates, like factor IX concentrates or one of its various
forms (Beriplex or factor VIII inhibitor bypassing activ-
ity), are considered “secondary components” and may be
acceptable to some Jehovah’s Witness patients [222].
Addition of factor IX concentrates may be most useful in
the highest risk Jehovah’s Witness patients.
d) Blood Salvage Interventions
EXPANDED USE OF RED CELL SALVAGE USING CENTRIFUGATION
Class IIb.
1. In high-risk patients with known malignancy who
require CPB, blood salvage using centrifugation of
blood salvaged from the operative field may be
considered since substantial data support benefit in
patients without malignancy, and new evidence
suggests worsened outcome when allogeneic trans-
fusion is required in patients with malignancy.
(Level of evidence B)
In 1986, the American Medical Association Council on
Scientific Affairs issued a statement regarding the safety
of blood salvage during cancer surgery [228]. At that
time, they advised against its use. Since then, 10 obser-
vational studies that included 476 patients who received
blood salvage during resection of multiple different tumor
types involving the liver [229–231], prostate [232–234],
uterus [235, 236], and urologic system [237, 238] support the
use of salvage of red cells using centrifugation in cancer
patients. In seven studies, a control group received no
transfusion, allogeneic transfusion, or preoperative autolo-
end of CPB is reasonable as part of a bl
agement program to minimize blood tr
(Level of evidence C)
2. Centrifugation instead of direct infusion o
pump blood is reasonable for minimizing
allogeneic RBC transfusion. (Level of evi
Most surgical teams reinfuse blood from t
poreal circuit (ECC) back into patients at the
as part of a blood conservation strategy. Cu
blood salvaging techniques exist: (1) direct
post-CPB circuit blood with no processing;
cessing of the circuit blood, either by centrifu
ultrafiltration, to remove either plasma com
water soluble components from blood before
Ann Thorac Surg FERRARIS
2011;91:944–82 STS BLOOD CONSERVATION REVISION
10 studi osservazionali su 476 pazienti
operati per diverse patologie tumorali
supportano l’uso del cell saver
molti reports indicano che i
pazienti che hanno ricevuto
trasfusioni allogeniche hanno un
maggior rischio di recidiva
mercoledì 26 novembre 14](https://image.slidesharecdn.com/romaint-160320122837/85/A-fresh-look-at-cell-salvage-57-320.jpg)
![2011 Update to The Society of Thoracic Surgeons
and the Society of Cardiovascular Anesthesiologists
Blood Conservation Clinical Practice Guidelines*
The Society of Thoracic Surgeons Blood Conservation Guideline Task Force:
Victor A. Ferraris, MD, PhD (Chair), Jeremiah R. Brown, PhD, George J. Despotis, MD,
John W. Hammon, MD, T. Brett Reece, MD, Sibu P. Saha, MD, MBA,
Howard K. Song, MD, PhD, and Ellen R. Clough, PhD
The Society of Cardiovascular Anesthesiologists Special Task Force on Blood Transfusion:
Linda J. Shore-Lesserson, MD, Lawrence T. Goodnough, MD, C. David Mazer, MD,
Aryeh Shander, MD, Mark Stafford-Smith, MD, and Jonathan Waters, MD
The International Consortium for Evidence Based Perfusion:
Robert A. Baker, PhD, Dip Perf, CCP (Aus), Timothy A. Dickinson, MS,
Daniel J. FitzGerald, CCP, LP, Donald S. Likosky, PhD, and Kenneth G. Shann, CCP
Division of Cardiovascular and Thoracic Surgery, University of Kentucky, Lexington, Kentucky (VAF, SPS), Department of
Anesthesiology, University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania (JW), Departments of Anesthesiology and Critical
Care Medicine, Englewood Hospital and Medical Center, Englewood, New Jersey (AS), Departments of Pathology and Medicine,
Stanford University School of Medicine, Stanford, California (LTG), Departments of Anesthesiology and Cardiothoracic Surgery,
Montefiore Medical Center, Bronx, New York (LJS-L, KGS), Departments of Anesthesiology, Immunology, and Pathology, Washington
University School of Medicine, St. Louis, Missouri (GJD), Dartmouth Institute for Health Policy and Clinical Practice, Section of
Cardiology, Dartmouth Medical School, Lebanon, New Hampshire (JRB), Department of Cardiothoracic Surgery, Wake Forest School of
Medicine, Winston-Salem, North Carolina (JWH), Department of Anesthesia, St. Michael’s Hospital, University of Toronto, Toronto,
Ontario (CDM), Cardiac Surgical Research Group, Flinders Medical Centre, South Australia, Australia (RAB), Department of Surgery,
Medicine, Community and Family Medicine, and the Dartmouth Institute for Health Policy and Clinical Practice, Dartmouth Medical
School, Hanover, New Hampshire (DSL), SpecialtyCare, Nashville, Tennessee (TAD), Department of Cardiac Surgery, Brigham and
Women’s Hospital, Harvard University, Boston, Massachusetts (DJF), Division of Cardiothoracic Surgery, Oregon Health and Science
University Medical Center, Portland, Oregon (HKS), Department of Cardiothoracic Surgery, University of Colorado Health Sciences
Center, Aurora, Colorado (TBR), Department of Anesthesiology, Duke University Medical Center, Durham, North Carolina (MS-S), and
The Society of Thoracic Surgeons, Chicago, Illinois (ERC)
Background. Practice guidelines reflect published liter- Methods. The search methods used in the current
pro-
bolic
ports
with
213].
t re-
lica-
that
om-
g, or
per-
ICU
Two
ship
pa-
volv-
diac
tar-
mbo-
able [227], and addition of factor concentrates augments
multiple other interventions. Fractionated factor concen-
trates, like factor IX concentrates or one of its various
forms (Beriplex or factor VIII inhibitor bypassing activ-
ity), are considered “secondary components” and may be
acceptable to some Jehovah’s Witness patients [222].
Addition of factor IX concentrates may be most useful in
the highest risk Jehovah’s Witness patients.
d) Blood Salvage Interventions
EXPANDED USE OF RED CELL SALVAGE USING CENTRIFUGATION
Class IIb.
1. In high-risk patients with known malignancy who
require CPB, blood salvage using centrifugation of
blood salvaged from the operative field may be
considered since substantial data support benefit in
patients without malignancy, and new evidence
suggests worsened outcome when allogeneic trans-
fusion is required in patients with malignancy.
(Level of evidence B)
In 1986, the American Medical Association Council on
Scientific Affairs issued a statement regarding the safety
of blood salvage during cancer surgery [228]. At that
time, they advised against its use. Since then, 10 obser-
vational studies that included 476 patients who received
blood salvage during resection of multiple different tumor
types involving the liver [229–231], prostate [232–234],
uterus [235, 236], and urologic system [237, 238] support the
use of salvage of red cells using centrifugation in cancer
patients. In seven studies, a control group received no
transfusion, allogeneic transfusion, or preoperative autolo-
end of CPB is reasonable as part of a bl
agement program to minimize blood tr
(Level of evidence C)
2. Centrifugation instead of direct infusion o
pump blood is reasonable for minimizing
allogeneic RBC transfusion. (Level of evi
Most surgical teams reinfuse blood from t
poreal circuit (ECC) back into patients at the
as part of a blood conservation strategy. Cu
blood salvaging techniques exist: (1) direct
post-CPB circuit blood with no processing;
cessing of the circuit blood, either by centrifu
ultrafiltration, to remove either plasma com
water soluble components from blood before
Ann Thorac Surg FERRARIS
2011;91:944–82 STS BLOOD CONSERVATION REVISION
10 studi osservazionali su 476 pazienti
operati per diverse patologie tumorali
supportano l’uso del cell saver
molti reports indicano che i
pazienti che hanno ricevuto
trasfusioni allogeniche hanno un
maggior rischio di recidiva
mercoledì 26 novembre 14](https://image.slidesharecdn.com/romaint-160320122837/85/A-fresh-look-at-cell-salvage-58-320.jpg)
![2011 Update to The Society of Thoracic Surgeons
and the Society of Cardiovascular Anesthesiologists
Blood Conservation Clinical Practice Guidelines*
The Society of Thoracic Surgeons Blood Conservation Guideline Task Force:
Victor A. Ferraris, MD, PhD (Chair), Jeremiah R. Brown, PhD, George J. Despotis, MD,
John W. Hammon, MD, T. Brett Reece, MD, Sibu P. Saha, MD, MBA,
Howard K. Song, MD, PhD, and Ellen R. Clough, PhD
The Society of Cardiovascular Anesthesiologists Special Task Force on Blood Transfusion:
Linda J. Shore-Lesserson, MD, Lawrence T. Goodnough, MD, C. David Mazer, MD,
Aryeh Shander, MD, Mark Stafford-Smith, MD, and Jonathan Waters, MD
The International Consortium for Evidence Based Perfusion:
Robert A. Baker, PhD, Dip Perf, CCP (Aus), Timothy A. Dickinson, MS,
Daniel J. FitzGerald, CCP, LP, Donald S. Likosky, PhD, and Kenneth G. Shann, CCP
Division of Cardiovascular and Thoracic Surgery, University of Kentucky, Lexington, Kentucky (VAF, SPS), Department of
Anesthesiology, University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania (JW), Departments of Anesthesiology and Critical
Care Medicine, Englewood Hospital and Medical Center, Englewood, New Jersey (AS), Departments of Pathology and Medicine,
Stanford University School of Medicine, Stanford, California (LTG), Departments of Anesthesiology and Cardiothoracic Surgery,
Montefiore Medical Center, Bronx, New York (LJS-L, KGS), Departments of Anesthesiology, Immunology, and Pathology, Washington
University School of Medicine, St. Louis, Missouri (GJD), Dartmouth Institute for Health Policy and Clinical Practice, Section of
Cardiology, Dartmouth Medical School, Lebanon, New Hampshire (JRB), Department of Cardiothoracic Surgery, Wake Forest School of
Medicine, Winston-Salem, North Carolina (JWH), Department of Anesthesia, St. Michael’s Hospital, University of Toronto, Toronto,
Ontario (CDM), Cardiac Surgical Research Group, Flinders Medical Centre, South Australia, Australia (RAB), Department of Surgery,
Medicine, Community and Family Medicine, and the Dartmouth Institute for Health Policy and Clinical Practice, Dartmouth Medical
School, Hanover, New Hampshire (DSL), SpecialtyCare, Nashville, Tennessee (TAD), Department of Cardiac Surgery, Brigham and
Women’s Hospital, Harvard University, Boston, Massachusetts (DJF), Division of Cardiothoracic Surgery, Oregon Health and Science
University Medical Center, Portland, Oregon (HKS), Department of Cardiothoracic Surgery, University of Colorado Health Sciences
Center, Aurora, Colorado (TBR), Department of Anesthesiology, Duke University Medical Center, Durham, North Carolina (MS-S), and
The Society of Thoracic Surgeons, Chicago, Illinois (ERC)
Background. Practice guidelines reflect published liter- Methods. The search methods used in the current
pro-
bolic
ports
with
213].
t re-
lica-
that
om-
g, or
per-
ICU
Two
ship
pa-
volv-
diac
tar-
mbo-
able [227], and addition of factor concentrates augments
multiple other interventions. Fractionated factor concen-
trates, like factor IX concentrates or one of its various
forms (Beriplex or factor VIII inhibitor bypassing activ-
ity), are considered “secondary components” and may be
acceptable to some Jehovah’s Witness patients [222].
Addition of factor IX concentrates may be most useful in
the highest risk Jehovah’s Witness patients.
d) Blood Salvage Interventions
EXPANDED USE OF RED CELL SALVAGE USING CENTRIFUGATION
Class IIb.
1. In high-risk patients with known malignancy who
require CPB, blood salvage using centrifugation of
blood salvaged from the operative field may be
considered since substantial data support benefit in
patients without malignancy, and new evidence
suggests worsened outcome when allogeneic trans-
fusion is required in patients with malignancy.
(Level of evidence B)
In 1986, the American Medical Association Council on
Scientific Affairs issued a statement regarding the safety
of blood salvage during cancer surgery [228]. At that
time, they advised against its use. Since then, 10 obser-
vational studies that included 476 patients who received
blood salvage during resection of multiple different tumor
types involving the liver [229–231], prostate [232–234],
uterus [235, 236], and urologic system [237, 238] support the
use of salvage of red cells using centrifugation in cancer
patients. In seven studies, a control group received no
transfusion, allogeneic transfusion, or preoperative autolo-
end of CPB is reasonable as part of a bl
agement program to minimize blood tr
(Level of evidence C)
2. Centrifugation instead of direct infusion o
pump blood is reasonable for minimizing
allogeneic RBC transfusion. (Level of evi
Most surgical teams reinfuse blood from t
poreal circuit (ECC) back into patients at the
as part of a blood conservation strategy. Cu
blood salvaging techniques exist: (1) direct
post-CPB circuit blood with no processing;
cessing of the circuit blood, either by centrifu
ultrafiltration, to remove either plasma com
water soluble components from blood before
Ann Thorac Surg FERRARIS
2011;91:944–82 STS BLOOD CONSERVATION REVISION
10 studi osservazionali su 476 pazienti
operati per diverse patologie tumorali
supportano l’uso del cell saver
molti reports indicano che i
pazienti che hanno ricevuto
trasfusioni allogeniche hanno un
maggior rischio di recidiva
due recenti metanalisi
suggeriscono che questo
rischio è doppio
mercoledì 26 novembre 14](https://image.slidesharecdn.com/romaint-160320122837/85/A-fresh-look-at-cell-salvage-59-320.jpg)
![2011 Update to The Society of Thoracic Surgeons
and the Society of Cardiovascular Anesthesiologists
Blood Conservation Clinical Practice Guidelines*
The Society of Thoracic Surgeons Blood Conservation Guideline Task Force:
Victor A. Ferraris, MD, PhD (Chair), Jeremiah R. Brown, PhD, George J. Despotis, MD,
John W. Hammon, MD, T. Brett Reece, MD, Sibu P. Saha, MD, MBA,
Howard K. Song, MD, PhD, and Ellen R. Clough, PhD
The Society of Cardiovascular Anesthesiologists Special Task Force on Blood Transfusion:
Linda J. Shore-Lesserson, MD, Lawrence T. Goodnough, MD, C. David Mazer, MD,
Aryeh Shander, MD, Mark Stafford-Smith, MD, and Jonathan Waters, MD
The International Consortium for Evidence Based Perfusion:
Robert A. Baker, PhD, Dip Perf, CCP (Aus), Timothy A. Dickinson, MS,
Daniel J. FitzGerald, CCP, LP, Donald S. Likosky, PhD, and Kenneth G. Shann, CCP
Division of Cardiovascular and Thoracic Surgery, University of Kentucky, Lexington, Kentucky (VAF, SPS), Department of
Anesthesiology, University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania (JW), Departments of Anesthesiology and Critical
Care Medicine, Englewood Hospital and Medical Center, Englewood, New Jersey (AS), Departments of Pathology and Medicine,
Stanford University School of Medicine, Stanford, California (LTG), Departments of Anesthesiology and Cardiothoracic Surgery,
Montefiore Medical Center, Bronx, New York (LJS-L, KGS), Departments of Anesthesiology, Immunology, and Pathology, Washington
University School of Medicine, St. Louis, Missouri (GJD), Dartmouth Institute for Health Policy and Clinical Practice, Section of
Cardiology, Dartmouth Medical School, Lebanon, New Hampshire (JRB), Department of Cardiothoracic Surgery, Wake Forest School of
Medicine, Winston-Salem, North Carolina (JWH), Department of Anesthesia, St. Michael’s Hospital, University of Toronto, Toronto,
Ontario (CDM), Cardiac Surgical Research Group, Flinders Medical Centre, South Australia, Australia (RAB), Department of Surgery,
Medicine, Community and Family Medicine, and the Dartmouth Institute for Health Policy and Clinical Practice, Dartmouth Medical
School, Hanover, New Hampshire (DSL), SpecialtyCare, Nashville, Tennessee (TAD), Department of Cardiac Surgery, Brigham and
Women’s Hospital, Harvard University, Boston, Massachusetts (DJF), Division of Cardiothoracic Surgery, Oregon Health and Science
University Medical Center, Portland, Oregon (HKS), Department of Cardiothoracic Surgery, University of Colorado Health Sciences
Center, Aurora, Colorado (TBR), Department of Anesthesiology, Duke University Medical Center, Durham, North Carolina (MS-S), and
The Society of Thoracic Surgeons, Chicago, Illinois (ERC)
Background. Practice guidelines reflect published liter- Methods. The search methods used in the current
pro-
bolic
ports
with
213].
t re-
lica-
that
om-
g, or
per-
ICU
Two
ship
pa-
volv-
diac
tar-
mbo-
able [227], and addition of factor concentrates augments
multiple other interventions. Fractionated factor concen-
trates, like factor IX concentrates or one of its various
forms (Beriplex or factor VIII inhibitor bypassing activ-
ity), are considered “secondary components” and may be
acceptable to some Jehovah’s Witness patients [222].
Addition of factor IX concentrates may be most useful in
the highest risk Jehovah’s Witness patients.
d) Blood Salvage Interventions
EXPANDED USE OF RED CELL SALVAGE USING CENTRIFUGATION
Class IIb.
1. In high-risk patients with known malignancy who
require CPB, blood salvage using centrifugation of
blood salvaged from the operative field may be
considered since substantial data support benefit in
patients without malignancy, and new evidence
suggests worsened outcome when allogeneic trans-
fusion is required in patients with malignancy.
(Level of evidence B)
In 1986, the American Medical Association Council on
Scientific Affairs issued a statement regarding the safety
of blood salvage during cancer surgery [228]. At that
time, they advised against its use. Since then, 10 obser-
vational studies that included 476 patients who received
blood salvage during resection of multiple different tumor
types involving the liver [229–231], prostate [232–234],
uterus [235, 236], and urologic system [237, 238] support the
use of salvage of red cells using centrifugation in cancer
patients. In seven studies, a control group received no
transfusion, allogeneic transfusion, or preoperative autolo-
end of CPB is reasonable as part of a bl
agement program to minimize blood tr
(Level of evidence C)
2. Centrifugation instead of direct infusion o
pump blood is reasonable for minimizing
allogeneic RBC transfusion. (Level of evi
Most surgical teams reinfuse blood from t
poreal circuit (ECC) back into patients at the
as part of a blood conservation strategy. Cu
blood salvaging techniques exist: (1) direct
post-CPB circuit blood with no processing;
cessing of the circuit blood, either by centrifu
ultrafiltration, to remove either plasma com
water soluble components from blood before
Ann Thorac Surg FERRARIS
2011;91:944–82 STS BLOOD CONSERVATION REVISION
10 studi osservazionali su 476 pazienti
operati per diverse patologie tumorali
supportano l’uso del cell saver
molti reports indicano che i
pazienti che hanno ricevuto
trasfusioni allogeniche hanno un
maggior rischio di recidiva
due recenti metanalisi
suggeriscono che questo
rischio è doppio
mercoledì 26 novembre 14](https://image.slidesharecdn.com/romaint-160320122837/85/A-fresh-look-at-cell-salvage-60-320.jpg)